The Conserved Coronavirus Macrodomain Promotes Virulence and Suppresses the Innate Immune Response during Severe Acute Respiratory Syndrome Coronavirus Infection
- PMID: 27965448
- PMCID: PMC5156301
- DOI: 10.1128/mBio.01721-16
The Conserved Coronavirus Macrodomain Promotes Virulence and Suppresses the Innate Immune Response during Severe Acute Respiratory Syndrome Coronavirus Infection
Abstract
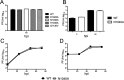
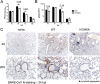
ADP-ribosylation is a common posttranslational modification that may have antiviral properties and impact innate immunity. To regulate this activity, macrodomain proteins enzymatically remove covalently attached ADP-ribose from protein targets. All members of the Coronavirinae, a subfamily of positive-sense RNA viruses, contain a highly conserved macrodomain within nonstructural protein 3 (nsp3). However, its function or targets during infection remain unknown. We identified several macrodomain mutations that greatly reduced nsp3's de-ADP-ribosylation activity in vitro Next, we created recombinant severe acute respiratory syndrome coronavirus (SARS-CoV) strains with these mutations. These mutations led to virus attenuation and a modest reduction of viral loads in infected mice, despite normal replication in cell culture. Further, macrodomain mutant virus elicited an early, enhanced interferon (IFN), interferon-stimulated gene (ISG), and proinflammatory cytokine response in mice and in a human bronchial epithelial cell line. Using a coinfection assay, we found that inclusion of mutant virus in the inoculum protected mice from an otherwise lethal SARS-CoV infection without reducing virus loads, indicating that the changes in innate immune response were physiologically significant. In conclusion, we have established a novel function for the SARS-CoV macrodomain that implicates ADP-ribose in the regulation of the innate immune response and helps to demonstrate why this domain is conserved in CoVs.
Importance: The macrodomain is a ubiquitous structural domain that removes ADP-ribose from proteins, reversing the activity of ADP-ribosyltransferases. All coronaviruses contain a macrodomain, suggesting that ADP-ribosylation impacts coronavirus infection. However, its function during infection remains unknown. Here, we found that the macrodomain is an important virulence factor for a highly pathogenic human CoV, SARS-CoV. Viruses with macrodomain mutations that abrogate its ability to remove ADP-ribose from protein were unable to cause lethal disease in mice. Importantly, the SARS-CoV macrodomain suppressed the innate immune response during infection. Our data suggest that an early innate immune response can protect mice from lethal disease. Understanding the mechanism used by this enzyme to promote disease will open up novel avenues for coronavirus therapies and give further insight into the role of macrodomains in viral pathogenesis.
Copyright © 2016 Fehr et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
