Modulation of Oligodendrocyte Differentiation by Mechanotransduction
- PMID: 27965541
- PMCID: PMC5126080
- DOI: 10.3389/fncel.2016.00277
Modulation of Oligodendrocyte Differentiation by Mechanotransduction
Abstract
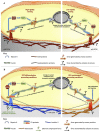
Oligodendrocytes (OLs) are responsible for the myelination of axons in the central nervous system (CNS). The differentiation of OLs encompasses several stages, through which cells undergo dramatic biochemical and morphological changes. OL differentiation is modulated by soluble factors (SFs)-such as growth factors and hormones-, known to be essential for each maturation stage. Besides SFs, insoluble factors such as extracellular matrix (ECM) proteins and other microenvironmental elements also play a pivotal role during OL differentiation. Recently, a growing number of studies were published concerning the effect of biophysical properties of the extracellular milieu on OL differentiation and myelination, showing the importance of ECM stiffness and topography, strain forces and spatial constraints. For instance, it was shown in vitro that OL differentiation and maturation is enhanced by substrates within the reported range of stiffness of the brain and that this effect is potentiated by the presence of merosin, whereas the myelination process is influenced by the diameter of axonal-like fibers. In this mini review article, we will discuss the effect of mechanical cues during OL differentiation and the possible molecular mechanisms involved in such regulation.
Keywords: differentiation; extracellular matrix; integrins; mechanobiology; mechanotransduction; myelination; neural stem cells; oligodendrocyte.
Figures

References
-
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., et al. . (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059. doi: 10.1016/j.cell.2013.07.042 - DOI - PubMed
-
- Arani A., Murphy M. C., Glaser K. J., Manduca A., Lake D. S., Kruse S. A., et al. . (2015). Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage 111, 59–64. doi: 10.1016/j.neuroimage.2015.02.016 - DOI - PMC - PubMed
-
- Arulmoli J., Pathak M. M., McDonnell L. P., Nourse J. L., Tombola F., Earthman J. C., et al. . (2015). Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci. Rep. 5:8499. doi: 10.1038/srep08499 - DOI - PMC - PubMed
-
- Bauer N. G., ffrench-Constant C. (2009). Physical forces in myelination and repair: a question of balance? J. Biol. 8:78. doi: 10.1186/jbiol169 - DOI - PMC - PubMed
-
- Bauer N. G., Richter-Landsberg C., Ffrench-Constant C. (2009). Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia 57, 1691–1705. doi: 10.1002/glia.20885 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

