An Exploratory Study of Spectroscopic Glutamatergic Correlates of Cortical Excitability in Depressed Adolescents
- PMID: 27965544
- PMCID: PMC5127083
- DOI: 10.3389/fncir.2016.00098
An Exploratory Study of Spectroscopic Glutamatergic Correlates of Cortical Excitability in Depressed Adolescents
Abstract
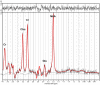
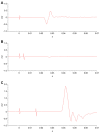
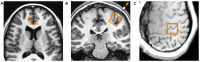
Introduction: Transcranial magnetic stimulation (TMS) research has suggested dysfunction in cortical glutamatergic systems in adolescent depression, while proton magnetic resonance spectroscopy (1H-MRS) studies have demonstrated deficits in concentrations of glutamatergic metabolites in depressed individuals in several cortical regions, including the anterior cingulate cortex (ACC). However, few studies have combined TMS and MRS methods to examine relationships between glutamatergic neurochemistry and excitatory and inhibitory neural functions, and none have utilized TMS-MRS methodology in clinical populations or in youth. This exploratory study aimed to examine relationships between TMS measures of cortical excitability and inhibition and concentrations of glutamatergic metabolites as measured by 1H-MRS in depressed adolescents. Methods: Twenty-four adolescents (aged 11-18 years) with depressive symptoms underwent TMS testing, which included measures of the resting motor threshold (RMT), cortical silent period (CSP), short-interval intracortical inhibition (SICI), and intracortical facilitation (ICF). Fourteen participants from the same sample also completed 1H-MRS in a 3 T MRI scanner after TMS testing. Glutamate + glutamine (Glx) concentrations were measured in medial ACC and left primary motor cortex voxels with a TE-optimized PRESS sequence. Metabolite concentrations were corrected for cerebrospinal fluid (CSF) after tissue segmentation. Pearson product-moment and Spearman rank-order correlations were calculated to assess relationships between TMS measures and [Glx]. Results: In the left primary motor cortex voxel, [Glx] had a significant positive correlation with the RMT. In the medial ACC voxel, [Glx] had significant positive correlations with ICF at the 10-ms and 20-ms interstimulus intervals (ISIs). Conclusion: These preliminary data implicate glutamate in cortical excitatory processes measured by TMS. Limitations included small sample size, lack of healthy control comparators, possible age- and sex-related effects, and observational nature of the study. Further research aimed at examining the relationship between glutamatergic metabolite concentrations measured through MRS and the excitatory and inhibitory physiology measured through TMS is warranted. Combined TMS-MRS methods show promise for future investigations of the pathophysiology of depression in adults as well as in children and adolescents.
Keywords: child and adolescent; cortical excitability; depression; glutamate; proton magnetic resonance spectroscopy; transcranial magnetic stimulation.
Figures

Similar articles
-
Cortical inhibitory and excitatory correlates of depression severity in children and adolescents.J Affect Disord. 2016 Jan 15;190:566-575. doi: 10.1016/j.jad.2015.10.020. Epub 2015 Oct 28. J Affect Disord. 2016. PMID: 26580570 Free PMC article.
-
Longitudinal assessment of 1H-MRS (GABA and Glx) and TMS measures of cortical inhibition and facilitation in the sensorimotor cortex.Exp Brain Res. 2019 Dec;237(12):3461-3474. doi: 10.1007/s00221-019-05691-z. Epub 2019 Nov 16. Exp Brain Res. 2019. PMID: 31734787
-
Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder.JAMA Psychiatry. 2013 Mar;70(3):291-9. doi: 10.1001/2013.jamapsychiatry.24. JAMA Psychiatry. 2013. PMID: 23303429
-
Cortical excitability on sleep deprivation measured by transcranial magnetic stimulation: A systematic review and meta-analysis.Brain Res Bull. 2025 Feb;221:111190. doi: 10.1016/j.brainresbull.2025.111190. Epub 2025 Jan 3. Brain Res Bull. 2025. PMID: 39756660
-
Pharmaco-transcranial magnetic stimulation studies of motor excitability.Handb Clin Neurol. 2013;116:387-97. doi: 10.1016/B978-0-444-53497-2.00032-2. Handb Clin Neurol. 2013. PMID: 24112911 Review.
Cited by
-
Motor cortex facilitation: a marker of attention deficit hyperactivity disorder co-occurrence in autism spectrum disorder.Transl Psychiatry. 2019 Nov 13;9(1):298. doi: 10.1038/s41398-019-0614-3. Transl Psychiatry. 2019. PMID: 31723120 Free PMC article.
-
Single Session Low Frequency Left Dorsolateral Prefrontal Transcranial Magnetic Stimulation Changes Neurometabolite Relationships in Healthy Humans.Front Hum Neurosci. 2018 Mar 26;12:77. doi: 10.3389/fnhum.2018.00077. eCollection 2018. Front Hum Neurosci. 2018. PMID: 29632477 Free PMC article.
-
A pilot spectroscopy study of adversity in adolescents.Biomark Neuropsychiatry. 2021 Dec;5:100043. doi: 10.1016/j.bionps.2021.100043. Epub 2021 Oct 2. Biomark Neuropsychiatry. 2021. PMID: 35783196 Free PMC article.
-
The Impact of Theta-Burst Stimulation on Cortical GABA and Glutamate in Treatment-Resistant Depression: A Surface-Based MRSI Analysis Approach.Front Mol Neurosci. 2022 Jul 13;15:913274. doi: 10.3389/fnmol.2022.913274. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35909445 Free PMC article.
-
Neurostructural Differences in Adolescents With Treatment-Resistant Depression and Treatment Effects of Transcranial Magnetic Stimulation.Int J Neuropsychopharmacol. 2022 Aug 16;25(8):619-630. doi: 10.1093/ijnp/pyac007. Int J Neuropsychopharmacol. 2022. PMID: 35089358 Free PMC article. Clinical Trial.
References
-
- Auer D. P., Pütz B., Kraft E., Lipinski B., Schill J., Holsboer F. (2000). Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry 47, 305–313. doi: 10.1016/s0006-3223(99)00159-6 - DOI - PubMed
-
- Bajbouj M., Lisanby S. H., Lang U. E., Danker-Hopfe H., Heuser I., Neu P. (2006). Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol. Psychiatry 59, 395–400. doi: 10.1016/j.biopsych.2005.07.036 - DOI - PubMed
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 57, 289–300.
-
- Bernstein I. H., Rush A. J., Trivedi M. H., Hughes C. W., Macleod L., Witte B. P., et al. . (2010). Psychometric properties of the Quick Inventory of Depressive Symptomatology in adolescents. Int. J. Methods Psychiatr. Res. 19, 185–194. doi: 10.1002/mpr.321 - DOI - PMC - PubMed
-
- Bestmann S., Feredoes E. (2013). Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present and future. Ann. N Y Acad. Sci. 1296, 11–30. doi: 10.1111/nyas.12110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

