Functional Genomics Identifies Tis21-Dependent Mechanisms and Putative Cancer Drug Targets Underlying Medulloblastoma Shh-Type Development
- PMID: 27965576
- PMCID: PMC5127835
- DOI: 10.3389/fphar.2016.00449
Functional Genomics Identifies Tis21-Dependent Mechanisms and Putative Cancer Drug Targets Underlying Medulloblastoma Shh-Type Development
Abstract
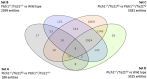
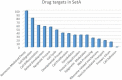
We have recently generated a novel medulloblastoma (MB) mouse model with activation of the Shh pathway and lacking the MB suppressor Tis21 (Patched1+/-/Tis21KO ). Its main phenotype is a defect of migration of the cerebellar granule precursor cells (GCPs). By genomic analysis of GCPs in vivo, we identified as drug target and major responsible of this defect the down-regulation of the promigratory chemokine Cxcl3. Consequently, the GCPs remain longer in the cerebellum proliferative area, and the MB frequency is enhanced. Here, we further analyzed the genes deregulated in a Tis21-dependent manner (Patched1+/-/Tis21 wild-type vs. Ptch1+/-/Tis21 knockout), among which are a number of down-regulated tumor inhibitors and up-regulated tumor facilitators, focusing on pathways potentially involved in the tumorigenesis and on putative new drug targets. The data analysis using bioinformatic tools revealed: (i) a link between the Shh signaling and the Tis21-dependent impairment of the GCPs migration, through a Shh-dependent deregulation of the clathrin-mediated chemotaxis operating in the primary cilium through the Cxcl3-Cxcr2 axis; (ii) a possible lineage shift of Shh-type GCPs toward retinal precursor phenotype, i.e., the neural cell type involved in group 3 MB; (iii) the identification of a subset of putative drug targets for MB, involved, among the others, in the regulation of Hippo signaling and centrosome assembly. Finally, our findings define also the role of Tis21 in the regulation of gene expression, through epigenetic and RNA processing mechanisms, influencing the fate of the GCPs.
Keywords: Sonic Hedgehog; cerebellar precursor cell; chemokines; drug target; medulloblastoma model; neural migration; primary cilium; retina.
Figures


Similar articles
-
Tis21-gene therapy inhibits medulloblastoma growth in a murine allograft model.PLoS One. 2018 Mar 14;13(3):e0194206. doi: 10.1371/journal.pone.0194206. eCollection 2018. PLoS One. 2018. PMID: 29538458 Free PMC article.
-
Tumor Growth in the High Frequency Medulloblastoma Mouse Model Ptch1+/-/Tis21KO Has a Specific Activation Signature of the PI3K/AKT/mTOR Pathway and Is Counteracted by the PI3K Inhibitor MEN1611.Front Oncol. 2021 Jul 30;11:692053. doi: 10.3389/fonc.2021.692053. eCollection 2021. Front Oncol. 2021. PMID: 34395258 Free PMC article.
-
Tis21 knock-out enhances the frequency of medulloblastoma in Patched1 heterozygous mice by inhibiting the Cxcl3-dependent migration of cerebellar neurons.J Neurosci. 2012 Oct 31;32(44):15547-64. doi: 10.1523/JNEUROSCI.0412-12.2012. J Neurosci. 2012. PMID: 23115191 Free PMC article.
-
Sonic Hedgehog Signaling in Cerebellar Development and Cancer.Front Cell Dev Biol. 2022 Apr 29;10:864035. doi: 10.3389/fcell.2022.864035. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573667 Free PMC article. Review.
-
Control of the Normal and Pathological Development of Neural Stem and Progenitor Cells by the PC3/Tis21/Btg2 and Btg1 Genes.J Cell Physiol. 2015 Dec;230(12):2881-90. doi: 10.1002/jcp.25038. J Cell Physiol. 2015. PMID: 25967096 Review.
Cited by
-
Tis21-gene therapy inhibits medulloblastoma growth in a murine allograft model.PLoS One. 2018 Mar 14;13(3):e0194206. doi: 10.1371/journal.pone.0194206. eCollection 2018. PLoS One. 2018. PMID: 29538458 Free PMC article.
-
Suppression of Medulloblastoma Lesions by Forced Migration of Preneoplastic Precursor Cells with Intracerebellar Administration of the Chemokine Cxcl3.Front Pharmacol. 2016 Dec 16;7:484. doi: 10.3389/fphar.2016.00484. eCollection 2016. Front Pharmacol. 2016. PMID: 28018222 Free PMC article.
-
Methylation Profiling of Medulloblastoma in a Clinical Setting Permits Sub-classification and Reveals New Outcome Predictions.Front Neurol. 2020 Mar 20;11:167. doi: 10.3389/fneur.2020.00167. eCollection 2020. Front Neurol. 2020. PMID: 32265819 Free PMC article.
-
Pyrido[2,3-d]pyrimidin-7(8H)-ones: Synthesis and Biomedical Applications.Molecules. 2019 Nov 16;24(22):4161. doi: 10.3390/molecules24224161. Molecules. 2019. PMID: 31744155 Free PMC article. Review.
-
Tumor Growth in the High Frequency Medulloblastoma Mouse Model Ptch1+/-/Tis21KO Has a Specific Activation Signature of the PI3K/AKT/mTOR Pathway and Is Counteracted by the PI3K Inhibitor MEN1611.Front Oncol. 2021 Jul 30;11:692053. doi: 10.3389/fonc.2021.692053. eCollection 2021. Front Oncol. 2021. PMID: 34395258 Free PMC article.
References
-
- Ahmed A. A., Lu Z., Jennings N. B., Etemadmoghadam D., Capalbo L., Jacamo R. O., et al. (2010). SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell 18, 109–121. 10.1016/j.ccr.2010.06.018 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

