Fatty Acid Regulation of Voltage- and Ligand-Gated Ion Channel Function
- PMID: 27965583
- PMCID: PMC5124694
- DOI: 10.3389/fphys.2016.00573
Fatty Acid Regulation of Voltage- and Ligand-Gated Ion Channel Function
Abstract
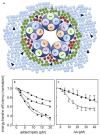
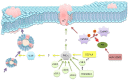
Free fatty acids (FFA) are essential components of the cell, where they play a key role in lipid and carbohydrate metabolism, and most particularly in cell membranes, where they are central actors in shaping the physicochemical properties of the lipid bilayer and the cellular adaptation to the environment. FFA are continuously being produced and degraded, and a feedback regulatory function has been attributed to their turnover. The massive increase observed under some pathological conditions, especially in brain, has been interpreted as a protective mechanism possibly operative on ion channels, which in some cases is of stimulatory nature and in other cases inhibitory. Here we discuss the correlation between the structure of FFA and their ability to modulate protein function, evaluating the influence of saturation/unsaturation, number of double bonds, and cis vs. trans isomerism. We further focus on the mechanisms of FFA modulation operating on voltage-gated and ligand-gated ion channel function, contrasting the still conflicting evidence on direct vs. indirect mechanisms of action.
Keywords: FFA; PUFA; VLCFA; cell-surface receptors; fatty acids; ion channels; ligand-gated channel.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

