Photoinactivation Using Visible Light Plus Water-Filtered Infrared-A (vis+wIRA) and Chlorine e6 (Ce6) Eradicates Planktonic Periodontal Pathogens and Subgingival Biofilms
- PMID: 27965635
- PMCID: PMC5124645
- DOI: 10.3389/fmicb.2016.01900
Photoinactivation Using Visible Light Plus Water-Filtered Infrared-A (vis+wIRA) and Chlorine e6 (Ce6) Eradicates Planktonic Periodontal Pathogens and Subgingival Biofilms
Abstract
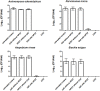
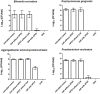
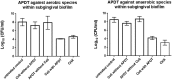
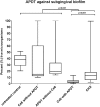
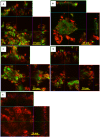
Alternative treatment methods for pathogens and microbial biofilms are required due to the widespread rise in antibiotic resistance. Antimicrobial photodynamic therapy (aPDT) has recently gained attention as a novel method to eradicate pathogens. The aim of this study was to evaluate the antimicrobial effects of a novel aPDT method using visible light (vis) and water infiltrated infrared A (wIRA) in combination with chlorine e6 (Ce6) against different periodontal pathogens in planktonic form and within in situ subgingival oral biofilms. Eight different periodontal pathogens were exposed to aPDT using vis+wIRA and 100 μg/ml Ce6 in planktonic culture. Additionally, pooled subgingival dental biofilm was also treated by aPDT and the number of viable cells determined as colony forming units (CFU). Live/dead staining was used in combination with confocal laser scanning microscopy to visualize and quantify antimicrobial effects within the biofilm samples. Untreated negative controls as well as 0.2% chlorhexidine-treated positive controls were used. All eight tested periodontal pathogens including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Eikenella corrodens, Actinomyces odontolyticus, Fusobacterium nucleatum, Parvimonas micra, Slackia exigua, and Atopobium rimae and the aPDT-treated subgingival biofilm were eliminated over the ranges of 3.43-8.34 and 3.91-4.28 log10 CFU in the log10 scale, respectively. Thus, aPDT showed bactericidal effects on the representative pathogens as well as on the in situ subgingival biofilm. The live/dead staining also revealed a significant reduction (33.45%) of active cells within the aPDT-treated subgingival biofilm. Taking the favorable tissue healing effects of vis+wIRA into consideration, the significant antimicrobial effects revealed in this study highlight the potential of aPDT using this light source in combination with Ce6 as an adjunctive method to treat periodontitis as well as periimplantitis. The present results encourage also the evaluation of this method for the treatment of caries and apical periodontitis.
Keywords: antibiotic resistance; antimicrobial photodynamic therapy; periodontitis; photosensitizer; subgingival biofilm.
Figures
References
-
- Alvarenga L. H., Prates R. A., Yoshimura T. M., Kato I. T., Suzuki L. C., Ribeiro M. S., et al. (2015). Aggregatibacter actinomycetemcomitans biofilm can be inactivated by methylene blue-mediated photodynamic therapy. Photodiagnosis Photodyn. Ther. 12 131–135. 10.1016/j.pdpdt.2014.10.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

