Critical Roles of Kupffer Cells in the Pathogenesis of Alcoholic Liver Disease: From Basic Science to Clinical Trials
- PMID: 27965666
- PMCID: PMC5126119
- DOI: 10.3389/fimmu.2016.00538
Critical Roles of Kupffer Cells in the Pathogenesis of Alcoholic Liver Disease: From Basic Science to Clinical Trials
Abstract
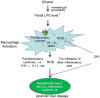
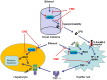
Alcoholic liver disease (ALD) encompasses a spectrum of liver injury ranging from steatosis to steatohepatitis, fibrosis, and finally cirrhosis. Accumulating evidences have demonstrated that Kupffer cells (KCs) play critical roles in the pathogenesis of both chronic and acute ALD. It has become clear that alcohol exposure can result in increased hepatic translocation of gut-sourced endotoxin/lipopolysaccharide, which is a strong M1 polarization inducer of KCs. The activated KCs then produce a large amount of reactive oxygen species (ROS), pro-inflammatory cytokines, and chemokines, which finally lead to liver injury. The critical roles of KCs and related inflammatory cascade in the pathogenesis of ALD make it a promising target in pharmaceutical drug developments for ALD treatment. Several drugs (such as rifaximin, pentoxifylline, and infliximab) have been evaluated or are under evaluation for ALD treatment in randomized clinical trials. Furthermore, screening pharmacological regulators for KCs toward M2 polarization may provide additional therapeutic agents. The combination of these potentially therapeutic drugs with hepatoprotective agents (such as zinc, melatonin, and silymarin) may bring encouraging results.
Keywords: Kupffer cells; alcoholic liver disease; cytochrome P4502E1; lipopolysaccharide; polarization; tumor necrosis factor α.
Figures


Similar articles
-
Kupffer Cells: Inflammation Pathways and Cell-Cell Interactions in Alcohol-Associated Liver Disease.Am J Pathol. 2020 Nov;190(11):2185-2193. doi: 10.1016/j.ajpath.2020.08.014. Epub 2020 Sep 11. Am J Pathol. 2020. PMID: 32919978 Free PMC article. Review.
-
M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease.Hepatology. 2014 Jan;59(1):130-42. doi: 10.1002/hep.26607. Epub 2013 Nov 20. Hepatology. 2014. PMID: 23832548
-
An endoplasmic reticulum protein, Nogo-B, facilitates alcoholic liver disease through regulation of kupffer cell polarization.Hepatology. 2017 May;65(5):1720-1734. doi: 10.1002/hep.29051. Epub 2017 Mar 22. Hepatology. 2017. PMID: 28090670 Free PMC article.
-
Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice.Hepatology. 2011 Oct;54(4):1217-26. doi: 10.1002/hep.24524. Epub 2011 Sep 6. Hepatology. 2011. PMID: 21735467
-
Cytokines in alcoholic liver disease.Arch Toxicol. 2012 Sep;86(9):1337-48. doi: 10.1007/s00204-012-0814-6. Epub 2012 Feb 25. Arch Toxicol. 2012. PMID: 22367091 Review.
Cited by
-
Advancements in the Alcohol-Associated Liver Disease Model.Biomolecules. 2022 Jul 27;12(8):1035. doi: 10.3390/biom12081035. Biomolecules. 2022. PMID: 36008929 Free PMC article. Review.
-
Up-regulation of miR-155 contributes to TNF-mediated hepatocyte apoptosis in acute liver failure.Turk J Gastroenterol. 2019 May;30(5):475-484. doi: 10.5152/tjg.2019.18159. Turk J Gastroenterol. 2019. PMID: 31061003 Free PMC article.
-
The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure.Nutrients. 2019 Apr 16;11(4):857. doi: 10.3390/nu11040857. Nutrients. 2019. PMID: 30995745 Free PMC article.
-
Gut Microbiota at the Intersection of Alcohol, Brain, and the Liver.J Clin Med. 2021 Feb 2;10(3):541. doi: 10.3390/jcm10030541. J Clin Med. 2021. PMID: 33540624 Free PMC article. Review.
-
Experimental In Vivo Toxicity Models for Alcohol Toxicity.Eurasian J Med. 2023 Dec 29;55(1):82-90. doi: 10.5152/eurasianjmed.2023.23345. Eurasian J Med. 2023. PMID: 39109811 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

