Activated IL-1RI Signaling Pathway Induces Th17 Cell Differentiation via Interferon Regulatory Factor 4 Signaling in Patients with Relapsing-Remitting Multiple Sclerosis
- PMID: 27965670
- PMCID: PMC5126112
- DOI: 10.3389/fimmu.2016.00543
Activated IL-1RI Signaling Pathway Induces Th17 Cell Differentiation via Interferon Regulatory Factor 4 Signaling in Patients with Relapsing-Remitting Multiple Sclerosis
Abstract
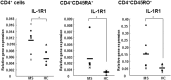
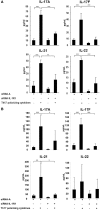
IL-1β plays a crucial role in the differentiation of human Th17 cells. We report here that IL-1RI expression is significantly increased in both naive and memory CD4+ T cells derived from relapsing-remitting multiple sclerosis (RR MS) patients in comparison to healthy controls. Interleukin 1 receptor (IL-1R)I expression is upregulated in the in vitro-differentiated Th17 cells from RR MS patients in comparison to the Th1 and Th2 cell subsets, indicating the role of IL-1R signaling in the Th17 cell differentiation in RR MS. When IL-1RI gene expression was silenced using siRNA, human naive CD4+ T cells cultured in the presence of Th17-polarizing cytokines had a significantly decreased expression of interleukin regulatory factor 4 (IRF4), RORc, IL-17A, IL-17F, IL-21, IL-22, and IL-23R genes, confirming that IL-1RI signaling induces Th17 cell differentiation. Since IL-1R gene expression silencing inhibited IRF4 expression and Th17 differentiation, and IRF4 gene expression silencing inhibited Th17 cell differentiation, our results indicate that IL-1RI induces human Th17 cell differentiation in an IRF4-dependant manner. Our study has identified that IL-1RI-mediated signaling pathway is constitutively activated, leading to an increased Th17 cell differentiation in IRF4-dependent manner in patients with RR MS.
Keywords: IL-1; IL-1R; IRF4; Th17 cells; multiple sclerosis.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

