Non-host Resistance Induced by the Xanthomonas Effector XopQ Is Widespread within the Genus Nicotiana and Functionally Depends on EDS1
- PMID: 27965697
- PMCID: PMC5127841
- DOI: 10.3389/fpls.2016.01796
Non-host Resistance Induced by the Xanthomonas Effector XopQ Is Widespread within the Genus Nicotiana and Functionally Depends on EDS1
Abstract
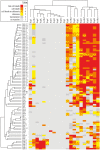
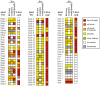
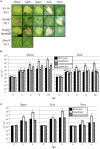
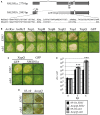
Most Gram-negative plant pathogenic bacteria translocate effector proteins (T3Es) directly into plant cells via a conserved type III secretion system, which is essential for pathogenicity in susceptible plants. In resistant plants, recognition of some T3Es is mediated by corresponding resistance (R) genes or R proteins and induces effector triggered immunity (ETI) that often results in programmed cell death reactions. The identification of R genes and understanding their evolution/distribution bears great potential for the generation of resistant crop plants. We focus on T3Es from Xanthomonas campestris pv. vesicatoria (Xcv), the causal agent of bacterial spot disease on pepper and tomato plants. Here, 86 Solanaceae lines mainly of the genus Nicotiana were screened for phenotypical reactions after Agrobacterium tumefaciens-mediated transient expression of 21 different Xcv effectors to (i) identify new plant lines for T3E characterization, (ii) analyze conservation/evolution of putative R genes and (iii) identify promising plant lines as repertoire for R gene isolation. The effectors provoked different reactions on closely related plant lines indicative of a high variability and evolution rate of potential R genes. In some cases, putative R genes were conserved within a plant species but not within superordinate phylogenetical units. Interestingly, the effector XopQ was recognized by several Nicotiana spp. lines, and Xcv infection assays revealed that XopQ is a host range determinant in many Nicotiana species. Non-host resistance against Xcv and XopQ recognition in N. benthamiana required EDS1, strongly suggesting the presence of a TIR domain-containing XopQ-specific R protein in these plant lines. XopQ is a conserved effector among most xanthomonads, pointing out the XopQ-recognizing RxopQ as candidate for targeted crop improvement.
Keywords: EDS1; ETI; Nicotiana benthamiana; Non-host resistance; Solanaceae; Xanthomonas; XopC; XopQ.
Figures


References
-
- Aarts N., Metz M., Holub E., Staskawicz B. J., Daniels M. J., Parker J. E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 95, 10306–10311. 10.1073/pnas.95.17.10306 - DOI - PMC - PubMed
-
- Bonas U., Schulte R., Fenselau S., Minsavage G. V., Staskawicz B. J., Stall R. E. (1991). Isolation of a gene cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol. Plant Microbe Interact. 4, 88.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

