Psychrophilic Lipase from Arctic Bacterium
- PMID: 27965754
- PMCID: PMC5131664
- DOI: 10.21315/tlsr2016.27.3.21
Psychrophilic Lipase from Arctic Bacterium
Abstract
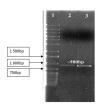
A lipase producer psychrophilic microorganism isolated from Arctic sample was studied. The genomic DNA of the isolate was extracted using modified CTAB method. Identification of the isolate by morphological and 16S rRNA sequence analysis revealed that the isolate is closely related to Arthrobacter gangotriensis (97% similarity). A. gangotriensis was determined as positive lipase producer based on the plate screening using specific and sensitive plate assay of Rhodamine B. The PCR result using Arthrobacter sp.'s full lipase gene sequence as the template primers emphasised a possible lipase gene at 900 bp band size. The gene is further cloned in a suitable vector system for expression of lipase.
Keywords: Arctic; Lipase; Psychrophiles.
Figures


References
-
- Bornscheuer UT, Bessler C, Srinivas R, Krishna SH. Optimizing lipases and related enzymes for efficient application. TRENDS in Biotechnology. 2002;20(10):433–437. http://dx.doi.org/10.1016/S0167-7799(02)02046-2. - DOI - PubMed
-
- Eltaweel MA, Rahman RNZRA, Salleh AB, Basri M. An organic solvent-stable lipase from Bacillus sp. strain 42. Annals of Microbiology. 2005;55(3):187–192.
-
- Gerday C, Aittaleb M, Arpigny JL, Baise EC, Garsoux G, Petressu I, Feller G. Psychrophilic enzymes: A thermodynamic challenge. Biochimica et Biophysica Acta. 1997;1342(2):119–131. http://dx.doi.org/10.1016/S0167-4838(97)00093-9. - DOI - PubMed
-
- Giudice AL, Michaud L, De Pascale D, De Domenico M, Prisco GDi, Fani R, Bruni V. Lipolytic activity of antarctic cold-adapted marine bacteria (Terra Nova Bay, Ross Sea). Journal of Applied Microbiology. 2006;101(5):1039–1048. http://dx.doi.org/10.1111/j.1365-2672.2006.03006.x. - DOI - PubMed
-
- Glogauer A, Viviane PM, Helisson F, Gustavo HC, Marcelo MS, Rose AM, David AM, Emanuel MS, Fabio OP, Nadia K. Identification and characterization of a new true lipase isolated through metagenomic approach. Journal of Microbiology Cell Factories. 2011;10(54) http://dx.doi.org/10.1186/1475-2859-10-54. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
