Survivors of intensive care with type 2 diabetes and the effect of shared care follow-up clinics: study protocol for the SWEET-AS randomised controlled feasibility study
- PMID: 27965877
- PMCID: PMC5153915
- DOI: 10.1186/s40814-016-0104-9
Survivors of intensive care with type 2 diabetes and the effect of shared care follow-up clinics: study protocol for the SWEET-AS randomised controlled feasibility study
Abstract
Background: Many patients who survive the intensive care unit (ICU) experience long-term complications such as peripheral neuropathy and nephropathy which represent a major source of morbidity and affect quality of life adversely. Similar pathophysiological processes occur frequently in ambulant patients with diabetes mellitus who have never been critically ill. Some 25 % of all adult ICU patients have diabetes, and it is plausible that ICU survivors with co-existing diabetes are at heightened risk of sequelae from their critical illness. ICU follow-up clinics are being progressively implemented based on the concept that interventions provided in these clinics will alleviate the burdens of survivorship. However, there is only limited information about their outcomes. The few existing studies have utilised the expertise of healthcare professionals primarily trained in intensive care and evaluated heterogenous cohorts. A shared care model with an intensivist- and diabetologist-led clinic for ICU survivors with type 2 diabetes represents a novel targeted approach that has not been evaluated previously. Prior to undertaking any definitive study, it is essential to establish the feasibility of this intervention.
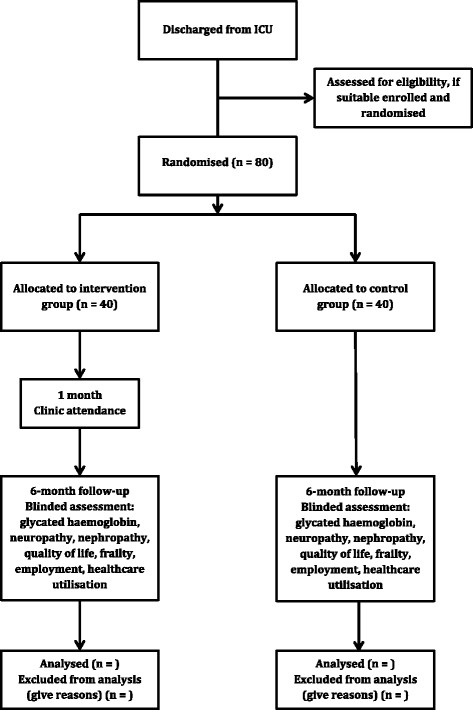
Methods: This will be a prospective, randomised, parallel, open-label feasibility study. Eligible patients will be approached before ICU discharge and randomised to the intervention (attending a shared care follow-up clinic 1 month after hospital discharge) or standard care. At each clinic visit, patients will be assessed independently by both an intensivist and a diabetologist who will provide screening and targeted interventions. Six months after discharge, all patients will be assessed by blinded assessors for glycated haemoglobin, peripheral neuropathy, cardiovascular autonomic neuropathy, nephropathy, quality of life, frailty, employment and healthcare utilisation. The primary outcome of this study will be the recruitment and retention at 6 months of all eligible patients.
Discussion: This study will provide preliminary data about the potential effects of critical illness on chronic glucose metabolism, the prevalence of microvascular complications, and the impact on healthcare utilisation and quality of life in intensive care survivors with type 2 diabetes. If feasibility is established and point estimates are indicative of benefit, funding will be sought for a larger, multi-centre study.
Trial registration: ANZCTR ACTRN12616000206426.
Keywords: Critical illness; Diabetes mellitus; Follow-up studies; Intensive care; Survivors.
Figures
Similar articles
-
Survivors of Intensive Care With Type 2 Diabetes and the Effect of Shared-Care Follow-Up Clinics: The SWEET-AS Randomized Controlled Pilot Study.Chest. 2021 Jan;159(1):174-185. doi: 10.1016/j.chest.2020.08.011. Epub 2020 Aug 12. Chest. 2021. PMID: 32800818 Clinical Trial.
-
Piloting an ICU follow-up clinic to improve health-related quality of life in ICU survivors after a prolonged intensive care stay (PINA): study protocol for a pilot randomised controlled trial.Pilot Feasibility Stud. 2021 Mar 30;7(1):90. doi: 10.1186/s40814-021-00796-1. Pilot Feasibility Stud. 2021. PMID: 33785064 Free PMC article.
-
Piloting an ICU follow-up clinic to improve health-related quality of life in ICU survivors after a prolonged intensive care stay (PINA): feasibility of a pragmatic randomised controlled trial.BMC Anesthesiol. 2023 Oct 14;23(1):344. doi: 10.1186/s12871-023-02255-1. BMC Anesthesiol. 2023. PMID: 37838669 Free PMC article. Clinical Trial.
-
Beyond the walls: a review of ICU clinics and their impact on patient outcomes after leaving hospital.Aust Crit Care. 2008 Feb;21(1):6-17. doi: 10.1016/j.aucc.2007.11.001. Aust Crit Care. 2008. PMID: 18206381 Review.
-
Reported burden on informal caregivers of ICU survivors: a literature review.Crit Care. 2016 Jan 21;20:16. doi: 10.1186/s13054-016-1185-9. Crit Care. 2016. PMID: 26792081 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials