CONSORT 2010 statement: extension to randomised pilot and feasibility trials
- PMID: 27965879
- PMCID: PMC5154046
- DOI: 10.1186/s40814-016-0105-8
CONSORT 2010 statement: extension to randomised pilot and feasibility trials
Abstract
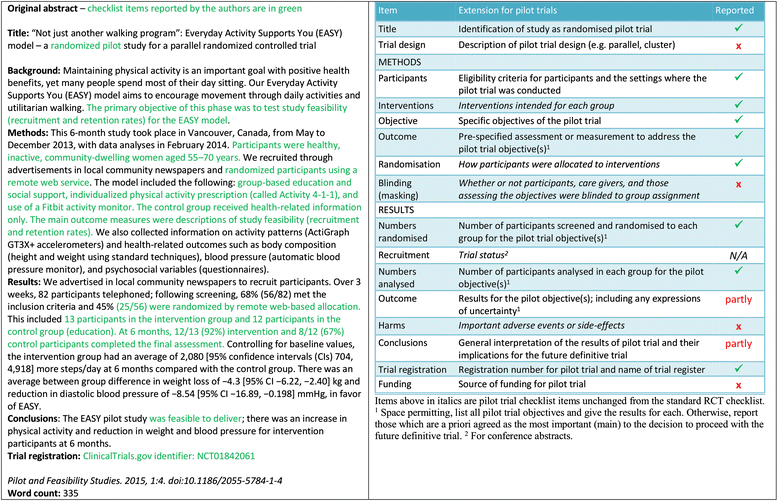
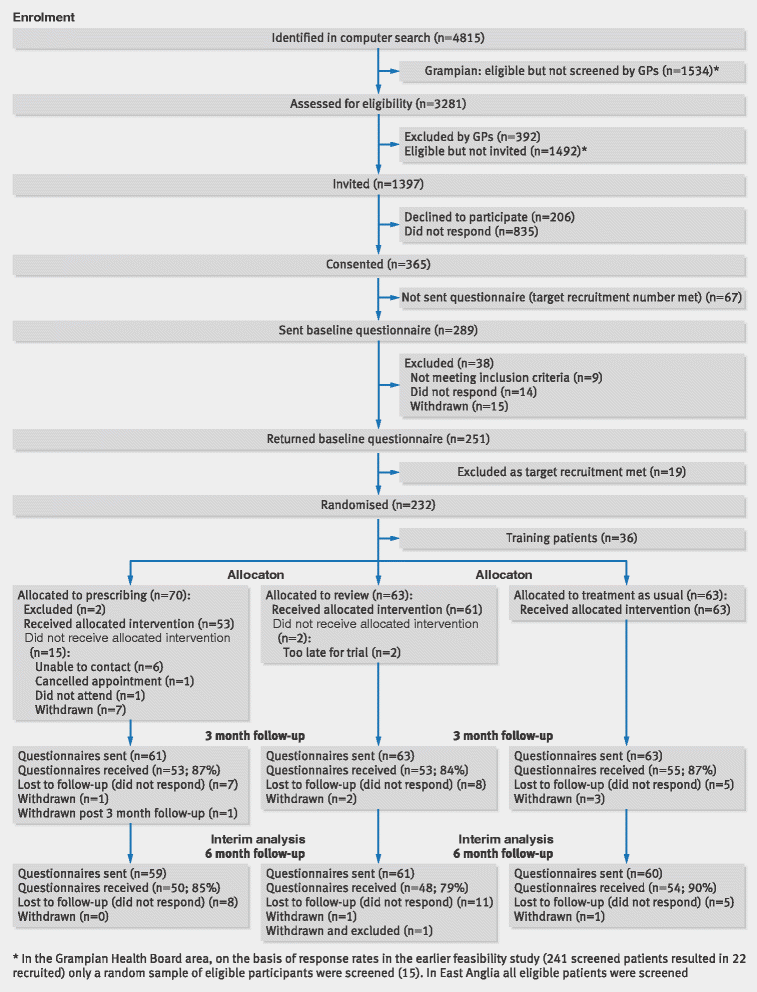
The Consolidated Standards of Reporting Trials (CONSORT) statement is a guideline designed to improve the transparency and quality of the reporting of randomised controlled trials (RCTs). In this article we present an extension to that statement for randomised pilot and feasibility trials conducted in advance of a future definitive RCT. The checklist applies to any randomised study in which a future definitive RCT, or part of it, is conducted on a smaller scale, regardless of its design (eg, cluster, factorial, crossover) or the terms used by authors to describe the study (eg, pilot, feasibility, trial, study). The extension does not directly apply to internal pilot studies built into the design of a main trial, non-randomised pilot and feasibility studies, or phase II studies, but these studies all have some similarities to randomised pilot and feasibility studies and so many of the principles might also apply. The development of the extension was motivated by the growing number of studies described as feasibility or pilot studies and by research that has identified weaknesses in their reporting and conduct. We followed recommended good practice to develop the extension, including carrying out a Delphi survey, holding a consensus meeting and research team meetings, and piloting the checklist. The aims and objectives of pilot and feasibility randomised studies differ from those of other randomised trials. Consequently, although much of the information to be reported in these trials is similar to those in randomised controlled trials (RCTs) assessing effectiveness and efficacy, there are some key differences in the type of information and in the appropriate interpretation of standard CONSORT reporting items. We have retained some of the original CONSORT statement items, but most have been adapted, some removed, and new items added. The new items cover how participants were identified and consent obtained; if applicable, the prespecified criteria used to judge whether or how to proceed with a future definitive RCT; if relevant, other important unintended consequences; implications for progression from pilot to future definitive RCT, including any proposed amendments; and ethical approval or approval by a research review committee confirmed with a reference number. This article includes the 26 item checklist, a separate checklist for the abstract, a template for a CONSORT flowchart for these studies, and an explanation of the changes made and supporting examples. We believe that routine use of this proposed extension to the CONSORT statement will result in improvements in the reporting of pilot trials. Editor's note: In order to encourage its wide dissemination this article is freely accessible on the BMJ and Pilot and Feasibility Studies journal websites.
Figures



References
-
- Turner L, Moher D, Shamseer L, et al. The influence of CONSORT on the quality of reporting of randomised controlled trials: an updated review. Trials. 2011;12(Suppl 1):A47. doi: 10.1186/1745-6215-12-S1-A47. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

