Diacylglycerol Kinases: Shaping Diacylglycerol and Phosphatidic Acid Gradients to Control Cell Polarity
- PMID: 27965956
- PMCID: PMC5126041
- DOI: 10.3389/fcell.2016.00140
Diacylglycerol Kinases: Shaping Diacylglycerol and Phosphatidic Acid Gradients to Control Cell Polarity
Abstract
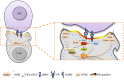
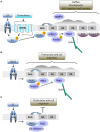
Diacylglycerol kinases (DGKs) terminate diacylglycerol (DAG) signaling and promote phosphatidic acid (PA) production. Isoform specific regulation of DGKs activity and localization allows DGKs to shape the DAG and PA gradients. The capacity of DGKs to constrain the areas of DAG signaling is exemplified by their role in defining the contact interface between T cells and antigen presenting cells: the immune synapse. Upon T cell receptor engagement, both DGK α and ζ metabolize DAG at the immune synapse thus constraining DAG signaling. Interestingly, their activity and localization are not fully redundant because DGKζ activity metabolizes the bulk of DAG in the cell, whereas DGKα limits the DAG signaling area localizing specifically at the periphery of the immune synapse. When DGKs terminate DAG signaling, the local PA production defines a new signaling domain, where PA recruits and activates a second wave of effector proteins. The best-characterized example is the role of DGKs in protrusion elongation and cell migration. Indeed, upon growth factor stimulation, several DGK isoforms, such as α, ζ, and γ, are recruited and activated at the plasma membrane. Here, local PA production controls cell migration by finely modulating cytoskeletal remodeling and integrin recycling. Interestingly, DGK-produced PA also controls the localization and activity of key players in cell polarity such as aPKC, Par3, and integrin β1. Thus, T cell polarization and directional migration may be just two instances of the general contribution of DGKs to the definition of cell polarity by local specification of membrane identity signaling.
Keywords: cell polarity; immune synapse; lipid domain; localization; migration.
Figures
References
-
- Alonso R., Mazzeo C., Rodriguez M. C., Marsh M., Fraile-Ramos A., Calvo V., et al. . (2011). Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 18, 1161–1173. 10.1038/cdd.2010.184 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

