Discovery and characterization of an F420-dependent glucose-6-phosphate dehydrogenase (Rh-FGD1) from Rhodococcus jostii RHA1
- PMID: 27966048
- PMCID: PMC5352752
- DOI: 10.1007/s00253-016-8038-y
Discovery and characterization of an F420-dependent glucose-6-phosphate dehydrogenase (Rh-FGD1) from Rhodococcus jostii RHA1
Abstract
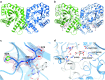
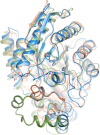
Cofactor F420, a 5-deazaflavin involved in obligatory hydride transfer, is widely distributed among archaeal methanogens and actinomycetes. Owing to the low redox potential of the cofactor, F420-dependent enzymes play a pivotal role in central catabolic pathways and xenobiotic degradation processes in these organisms. A physiologically essential deazaflavoenzyme is the F420-dependent glucose-6-phosphate dehydrogenase (FGD), which catalyzes the reaction F420 + glucose-6-phosphate → F420H2 + 6-phospho-gluconolactone. Thereby, FGDs generate the reduced F420 cofactor required for numerous F420H2-dependent reductases, involved e.g., in the bioreductive activation of the antitubercular prodrugs pretomanid and delamanid. We report here the identification, production, and characterization of three FGDs from Rhodococcus jostii RHA1 (Rh-FGDs), being the first experimental evidence of F420-dependent enzymes in this bacterium. The crystal structure of Rh-FGD1 has also been determined at 1.5 Å resolution, showing a high similarity with FGD from Mycobacterium tuberculosis (Mtb) (Mtb-FGD1). The cofactor-binding pocket and active-site catalytic residues are largely conserved in Rh-FGD1 compared with Mtb-FGD1, except for an extremely flexible insertion region capping the active site at the C-terminal end of the TIM-barrel, which also markedly differs from other structurally related proteins. The role of the three positively charged residues (Lys197, Lys258, and Arg282) constituting the binding site of the substrate phosphate moiety was experimentally corroborated by means of mutagenesis study. The biochemical and structural data presented here provide the first step towards tailoring Rh-FGD1 into a more economical biocatalyst, e.g., an F420-dependent glucose dehydrogenase that requires a cheaper cosubstrate and can better match the demands for the growing applications of F420H2-dependent reductases in industry and bioremediation.
Keywords: Deazaflavoenzymes; F420; Glucose-6-phosphate dehydrogenase; Rhodococcus.
Conflict of interest statement
Funding
This study was funded by a Ubbo Emmius scholarship from the University of Groningen, the Netherlands (awarded to QTN), and the European Community’s Seventh Framework Programme (FP7/2007−2013) under BioStruct-X (Grants 7551 and 10205).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Figures


Similar articles
-
Glucose-6-phosphate dehydrogenase and its 3D structures from crystallography and electron cryo-microscopy.Acta Crystallogr F Struct Biol Commun. 2024 Oct 1;80(Pt 10):236-251. doi: 10.1107/S2053230X24008112. Epub 2024 Sep 11. Acta Crystallogr F Struct Biol Commun. 2024. PMID: 39259139 Free PMC article. Review.
-
Coenzyme F420-Dependent Glucose-6-Phosphate Dehydrogenase-Coupled Polyglutamylation of Coenzyme F420 in Mycobacteria.J Bacteriol. 2018 Nov 6;200(23):e00375-18. doi: 10.1128/JB.00375-18. Print 2018 Dec 1. J Bacteriol. 2018. PMID: 30249701 Free PMC article.
-
Expression and purification of Mycobacterium tuberculosis F420-dependent glucose-6-phosphate dehydrogenase enzyme using Escherichia coli.Protein Expr Purif. 2025 Apr;228:106650. doi: 10.1016/j.pep.2024.106650. Epub 2025 Jan 6. Protein Expr Purif. 2025. PMID: 39778697
-
Investigating the Reaction Mechanism of F420-Dependent Glucose-6-phosphate Dehydrogenase from Mycobacterium tuberculosis: Kinetic Analysis of the Wild-Type and Mutant Enzymes.Biochemistry. 2016 Oct 4;55(39):5566-5577. doi: 10.1021/acs.biochem.6b00638. Epub 2016 Sep 20. Biochemistry. 2016. PMID: 27603793
-
Physiology, Biochemistry, and Applications of F420- and Fo-Dependent Redox Reactions.Microbiol Mol Biol Rev. 2016 Apr 27;80(2):451-93. doi: 10.1128/MMBR.00070-15. Print 2016 Jun. Microbiol Mol Biol Rev. 2016. PMID: 27122598 Free PMC article. Review.
Cited by
-
Glucose-6-phosphate dehydrogenase and its 3D structures from crystallography and electron cryo-microscopy.Acta Crystallogr F Struct Biol Commun. 2024 Oct 1;80(Pt 10):236-251. doi: 10.1107/S2053230X24008112. Epub 2024 Sep 11. Acta Crystallogr F Struct Biol Commun. 2024. PMID: 39259139 Free PMC article. Review.
-
Editorial: Actinobacteria, a Source of Biocatalytic Tools.Front Microbiol. 2019 Apr 16;10:800. doi: 10.3389/fmicb.2019.00800. eCollection 2019. Front Microbiol. 2019. PMID: 31040839 Free PMC article. No abstract available.
-
Genome-Based Insights into the Production of Carotenoids by Antarctic Bacteria, Planococcus sp. ANT_H30 and Rhodococcus sp. ANT_H53B.Molecules. 2020 Sep 23;25(19):4357. doi: 10.3390/molecules25194357. Molecules. 2020. PMID: 32977394 Free PMC article.
-
Characterization of a Novel Oxidative Stress Responsive Transcription Regulator in Mycobacterium bovis.Biomedicines. 2024 Aug 16;12(8):1872. doi: 10.3390/biomedicines12081872. Biomedicines. 2024. PMID: 39200336 Free PMC article.
-
Peroxisomes and Oxidative Stress: Their Implications in the Modulation of Cellular Immunity During Mycobacterial Infection.Front Microbiol. 2019 Jun 14;10:1121. doi: 10.3389/fmicb.2019.01121. eCollection 2019. Front Microbiol. 2019. PMID: 31258517 Free PMC article. Review.
References
-
- Ahmed FH, Carr PD, Lee BM, Afriat-Jurnou L, Mohamed AE, Hong N, Flanagan J, Taylor MC, Greening C, Jackson CJ. Sequence–structure–function classification of a catalytically diverse oxidoreductase superfamily in Mycobacteria. J Mol Biol. 2015;427:3554–3571. doi: 10.1016/j.jmb.2015.09.021. - DOI - PubMed
-
- Aufhammer SW, Warkentin E, Ermler U, Hagemeier CH, Thauer RK, Shima S. Crystal structure of methylenetetrahydromethanopterin reductase (Mer) in complex with coenzyme F420: architecture of the F420/FMN binding site of enzymes within the nonprolyl cis-peptide containing bacterial luciferase family. Protein Sci. 2005;14:1840–1849. doi: 10.1110/ps.041289805. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

