ImmunoPET Imaging of Murine CD4+ T Cells Using Anti-CD4 Cys-Diabody: Effects of Protein Dose on T Cell Function and Imaging
- PMID: 27966069
- PMCID: PMC5524218
- DOI: 10.1007/s11307-016-1032-z
ImmunoPET Imaging of Murine CD4+ T Cells Using Anti-CD4 Cys-Diabody: Effects of Protein Dose on T Cell Function and Imaging
Abstract
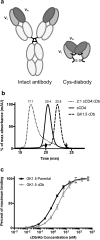
Purpose: Molecular imaging of CD4+ T cells throughout the body has implications for monitoring autoimmune disease and immunotherapy of cancer. Given the key role of these cells in regulating immunity, it is important to develop a biologically inert probe. GK1.5 cys-diabody (cDb), a previously developed anti-mouse CD4 antibody fragment, was tested at different doses to assess its effects on positron emission tomography (PET) imaging and CD4+ T cell viability, proliferation, CD4 expression, and function.
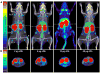
Procedures: The effect of protein dose on image contrast (lymphoid tissue-to-muscle ratio) was assessed by administering different amounts of 89Zr-labeled GK1.5 cDb to mice followed by PET imaging and ex vivo biodistribution analysis. To assess impact of GK1.5 cDb on T cell biology, GK1.5 cDb was incubated with T cells in vitro or administered intravenously to C57BL/6 mice at multiple protein doses. CD4 expression and T cell proliferation were analyzed with flow cytometry and cytokines were assayed.
Results: For immunoPET imaging, the lowest protein dose of 2 μg of 89Zr-labeled GK1.5 cDb resulted in significantly higher % injected dose/g in inguinal lymph nodes (ILN) and spleen compared to the 12-μg protein dose. In vivo administration of GK1.5 cDb at the high dose of 40 μg caused a transient decrease in CD4 expression in spleen, blood, lymph nodes, and thymus, which recovered within 3 days postinjection; this effect was reduced, although not abrogated, when 2 μg was administered. Proliferation was inhibited in vivo in ILN but not the spleen by injection of 40 μg GK1.5 cDb. Concentrations of GK1.5 cDb in excess of 25 nM significantly inhibited CD4+ T cell proliferation and interferon-γ production in vitro. Overall, using low-dose GK1.5 cDb minimized biological effects on CD4+ T cells.
Conclusions: Low-dose GK1.5 cDb yields high-contrast immunoPET images with minimal effects on T cell biology in vitro and in vivo and may be a useful tool for investigating CD4+ T cells in the context of preclinical disease models. Future approaches to minimizing biological effects may include the creation of monovalent fragments or selecting anti-CD4 antibodies which target alternative epitopes.
Keywords: Antibody engineering; CD4; Diabody; ImmunoPET; Lymphocytes; Positron emission tomography; T cell function; T cells; Zirconium-89.
Conflict of interest statement
A. M. W. is a stockholder in and consultant to ImaginAb, Inc.
Figures
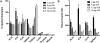

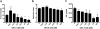
Similar articles
-
Reengineered Anti-CD4 Cys-diabody Variants for 89Zr-immunoPET of CD4+ T Cells in Immunocompetent Mice.Mol Imaging Biol. 2025 Aug 7. doi: 10.1007/s11307-025-02043-y. Online ahead of print. Mol Imaging Biol. 2025. PMID: 40775564
-
Immuno-PET in Inflammatory Bowel Disease: Imaging CD4-Positive T Cells in a Murine Model of Colitis.J Nucl Med. 2018 Jun;59(6):980-985. doi: 10.2967/jnumed.117.199075. Epub 2018 Jan 11. J Nucl Med. 2018. PMID: 29326360 Free PMC article.
-
Immuno-PET of Murine T Cell Reconstitution Postadoptive Stem Cell Transplantation Using Anti-CD4 and Anti-CD8 Cys-Diabodies.J Nucl Med. 2015 Aug;56(8):1258-64. doi: 10.2967/jnumed.114.153338. Epub 2015 May 7. J Nucl Med. 2015. PMID: 25952734 Free PMC article.
-
Advances and challenges in immunoPET methodology.Front Nucl Med. 2024 Feb 19;4:1360710. doi: 10.3389/fnume.2024.1360710. eCollection 2024. Front Nucl Med. 2024. PMID: 39355220 Free PMC article. Review.
-
ImmunoPET Directed to the Brain: A New Tool for Preclinical and Clinical Neuroscience.Biomolecules. 2023 Jan 13;13(1):164. doi: 10.3390/biom13010164. Biomolecules. 2023. PMID: 36671549 Free PMC article. Review.
Cited by
-
Radionuclide-based molecular imaging allows CAR-T cellular visualization and therapeutic monitoring.Theranostics. 2021 May 3;11(14):6800-6817. doi: 10.7150/thno.56989. eCollection 2021. Theranostics. 2021. PMID: 34093854 Free PMC article. Review.
-
In vivo Imaging Technologies to Monitor the Immune System.Front Immunol. 2020 Jun 2;11:1067. doi: 10.3389/fimmu.2020.01067. eCollection 2020. Front Immunol. 2020. PMID: 32582173 Free PMC article. Review.
-
Imaging of T-cell Responses in the Context of Cancer Immunotherapy.Cancer Immunol Res. 2021 May;9(5):490-502. doi: 10.1158/2326-6066.CIR-20-0678. Cancer Immunol Res. 2021. PMID: 33941536 Free PMC article. Review.
-
Promise and challenges of clinical non-invasive T-cell tracking in the era of cancer immunotherapy.EJNMMI Res. 2022 Jan 31;12(1):5. doi: 10.1186/s13550-022-00877-z. EJNMMI Res. 2022. PMID: 35099641 Free PMC article. Review.
-
In Vivo Measurement of Granzyme Proteolysis from Activated Immune Cells with PET.ACS Cent Sci. 2021 Oct 27;7(10):1638-1649. doi: 10.1021/acscentsci.1c00529. Epub 2021 Sep 2. ACS Cent Sci. 2021. PMID: 34729407 Free PMC article.
References
-
- Steinhoff K, Pierer M, Siegert J, et al. Visualizing inflammation activity in rheumatoid arthritis with Tc-99 m anti-CD4-mAb fragment scintigraphy. Nucl Med Biol. 2014;41:350–4. - PubMed
-
- Becker W, Emmrich F, Horneff G, et al. Imaging rheumatoid arthritis specifically with technetium 99m CD4-specific (T-helper lymphocytes) antibodies. Eur J Nucl Med. 1990;17:156–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

