Signaling pathways underlying the antidepressant-like effect of inosine in mice
- PMID: 27966087
- PMCID: PMC5432480
- DOI: 10.1007/s11302-016-9551-2
Signaling pathways underlying the antidepressant-like effect of inosine in mice
Abstract
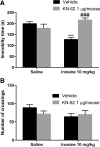
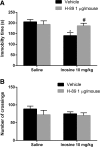
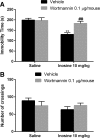
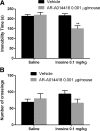
Inosine is a purine nucleoside formed by the breakdown of adenosine that elicits an antidepressant-like effect in mice through activation of adenosine A1 and A2A receptors. However, the signaling pathways underlying this effect are largely unknown. To address this issue, the present study investigated the influence of extracellular-regulated protein kinase (ERK)1/2, Ca2+/calmoduline-dependent protein kinase (CaMKII), protein kinase A (PKA), phosphoinositide 3-kinase (PI3K)/Akt, and glycogen synthase kinase 3beta (GSK-3β) modulation in the antiimmobility effect of inosine in the tail suspension test (TST) in mice. In addition, we attempted to verify if inosine treatment was capable of altering the immunocontent and phosphorylation of the transcription factor cyclic adenosine monophosphatate (cAMP) response-binding element protein (CREB) in mouse prefrontal cortex and hippocampus. Intracerebroventricular administration of U0126 (5 μg/mouse, MEK1/2 inhibitor), KN-62 (1 μg/mouse, CaMKII inhibitor), H-89 (1 μg/mouse, PKA inhibitor), and wortmannin (0.1 μg/mouse, PI3K inhibitor) prevented the antiimmobility effect of inosine (10 mg/kg, intraperitoneal (i.p.)) in the TST. Also, administration of a sub-effective dose of inosine (0.1 mg/kg, i.p.) in combination with a sub-effective dose of AR-A014418 (0.001 μg/mouse, GSK-3β inhibitor) induced a synergic antidepressant-like effect. None of the treatments altered locomotor activity of mice. Moreover, 24 h after a single administration of inosine (10 mg/kg, i.p.), CREB phosphorylation was increased in the hippocampus. Our findings provided new evidence that the antidepressant-like effect of inosine in the TST involves the activation of PKA, PI3K/Akt, ERK1/2, and CaMKII and the inhibition of GSK-3β. These results contribute to the comprehension of the mechanisms underlying the purinergic system modulation and indicate the intracellular signaling pathways involved in the antidepressant-like effect of inosine in a preclinical test of depression.
Keywords: CREB; Depression; Inosine; Protein kinases; Signaling pathways.
Conflict of interest statement
Conflicts of interest
Filipe Marques Gonçalves declares that he has no conflict of interest.
Vivian Binder Neis declares that she has no conflict of interest.
Débora Kurrle Rieger declares that she has no conflict of interest.
Mark William Lopes declares that he has no conflict of interest.
Isabela A. Heinrich declares that she has no conflict of interest.
Ana Paula Costa declares that she has no conflict of interest.
Ana Lúcia S. Rodrigues declares that she has no conflict of interest.
Manuella P. Kaster declares that she has no conflict of interest.
Rodrigo Bainy Leal declares that he has no conflict of interest.
Ethical approval
The procedures in this study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all the experimental protocols were approved by the Institutional Ethics Committee (protocol number PP00772). All efforts were made to minimize animal suffering and to reduce the number of animals used in the experiments.
Figures


Similar articles
-
Antidepressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Jun 3;59:59-67. doi: 10.1016/j.pnpbp.2015.01.008. Epub 2015 Jan 17. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25600102
-
Involvement of PI3K/Akt/GSK-3β and mTOR in the antidepressant-like effect of atorvastatin in mice.J Psychiatr Res. 2016 Nov;82:50-7. doi: 10.1016/j.jpsychires.2016.07.004. Epub 2016 Jul 7. J Psychiatr Res. 2016. PMID: 27468164
-
The antidepressant-like effect of guanosine is dependent on GSK-3β inhibition and activation of MAPK/ERK and Nrf2/heme oxygenase-1 signaling pathways.Purinergic Signal. 2019 Dec;15(4):491-504. doi: 10.1007/s11302-019-09681-2. Epub 2019 Nov 25. Purinergic Signal. 2019. PMID: 31768875 Free PMC article.
-
Antidepressant-like effect of ascorbic acid is associated with the modulation of mammalian target of rapamycin pathway.J Psychiatr Res. 2014 Jan;48(1):16-24. doi: 10.1016/j.jpsychires.2013.10.014. Epub 2013 Oct 29. J Psychiatr Res. 2014. PMID: 24209999
-
Inosine as a Tool to Understand and Treat Central Nervous System Disorders: A Neglected Actor?Front Neurosci. 2021 Aug 24;15:703783. doi: 10.3389/fnins.2021.703783. eCollection 2021. Front Neurosci. 2021. PMID: 34504414 Free PMC article. Review.
Cited by
-
Glutamatergic system and mTOR-signaling pathway participate in the antidepressant-like effect of inosine in the tail suspension test.J Neural Transm (Vienna). 2017 Oct;124(10):1227-1237. doi: 10.1007/s00702-017-1753-4. Epub 2017 Jul 10. J Neural Transm (Vienna). 2017. PMID: 28695335
-
Mitochondria: A Connecting Link in the Major Depressive Disorder Jigsaw.Curr Neuropharmacol. 2019;17(6):550-562. doi: 10.2174/1570159X16666180302120322. Curr Neuropharmacol. 2019. PMID: 29512466 Free PMC article. Review.
-
Purinergic Signalling: Therapeutic Developments.Front Pharmacol. 2017 Sep 25;8:661. doi: 10.3389/fphar.2017.00661. eCollection 2017. Front Pharmacol. 2017. PMID: 28993732 Free PMC article. Review.
-
The ERK Pathway: Molecular Mechanisms and Treatment of Depression.Mol Neurobiol. 2019 Sep;56(9):6197-6205. doi: 10.1007/s12035-019-1524-3. Epub 2019 Feb 9. Mol Neurobiol. 2019. PMID: 30737641 Free PMC article. Review.
-
Tumour initiated purinergic signalling promotes cardiomyocyte RBFOX1 degradation and cardiotoxicity from DNA damaging anticancer agents.Nat Commun. 2025 Jul 25;16(1):6861. doi: 10.1038/s41467-025-62172-4. Nat Commun. 2025. PMID: 40715150 Free PMC article.
References
-
- Ruhe HG, van Rooijen G, Spijker J, Peeters FP, Schene AH. Staging methods for treatment resistant depression. A systematic review. J Affect Disord. 2011;137(1–3):35–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

