Optical properties of biomimetic probes engineered from erythrocytes
- PMID: 27966473
- PMCID: PMC5189990
- DOI: 10.1088/1361-6528/28/3/035101
Optical properties of biomimetic probes engineered from erythrocytes
Abstract
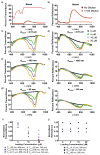
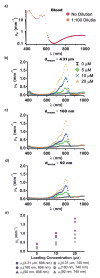
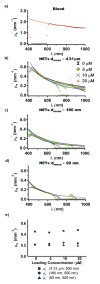
Light-activated theranostic materials offer a potential platform for optical imaging and phototherapeutic applications. We have engineered constructs derived from erythrocytes, which can be doped with the FDA-approved near infrared (NIR) chromophore, indocyanine green (ICG). We refer to these constructs as NIR erythrocyte-mimicking transducers (NETs). Herein, we investigated the effects of changing the NETs mean diameter from micron- (≈4 μm) to nano- (≈90 nm) scale, and the ICG concentration utilized in the fabrication of NETs from 5 to 20 μM on the resulting absorption and scattering characteristics of the NETs. Our approach consisted of integrating sphere-based measurements of light transmittance and reflectance, and subsequent utilization of these measurements in an inverse adding-doubling algorithm to estimate the absorption (μ a) and reduced scattering (μ s') coefficients of these NETs. For a given NETs diameter, values of μ a increased over the approximate spectral band of 630-860 nm with increasing ICG concentration. Micron-sized NETs produced the highest peak value of μ a when using ICG concentrations of 10 and 20 μM, and showed increased values of μ s' as compared to nano-sized NETs. Spectral profiles of μ s' for these NETs showed a trend consistent with Mie scattering behavior for spherical objects. For all NETs investigated, changing the ICG concentration minimally affected the scattering characteristics. A Monte Carlo-based model of light distribution showed that the presence of these NETs enhanced the fluence levels within simulated blood vessels. These results provide important data towards determining the appropriate light dosimetry parameters for an intended light-based biomedical application of NETs.
Figures
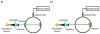
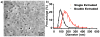

References
-
- Kim H, Chung K, Lee S, Kim DH, Lee H. Near-infrared light-responsive nano-materials for cancer theranostics. WIREs Nanomed Nanobiotechnol. 2016;8:23–25. - PubMed
-
- Cheng L, Yuan C, Shen S, Yi X, Gong H, Yang K, Liu Z. Bottom-up synthesis of metal-ion-doped WS2 nanoflakes for cancer theranostics. ACS Nano. 2015;9:11090–11101. - PubMed
-
- Lee M-Y, Lee C, Jung HS, Jeon M, Kim KS, Yun SH, Kim C, Hahn SK. Biodegradable photonic melanoidin for theranostic applications. ACS Nano. 2016;10:822–831. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
