Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone
- PMID: 27966526
- PMCID: PMC5171795
- DOI: 10.1038/ncomms13885
Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone
Erratum in
-
Author Correction: Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone.Nat Commun. 2023 Oct 19;14(1):6620. doi: 10.1038/s41467-023-42299-y. Nat Commun. 2023. PMID: 37857608 Free PMC article. No abstract available.
Abstract
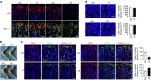
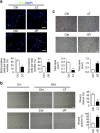
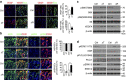
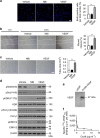
Communication between osteoblasts and endothelial cells (ECs) is essential for bone turnover, but the molecular mechanisms of such communication are not well defined. Here we identify Cxcl9 as an angiostatic factor secreted by osteoblasts in the bone marrow microenvironment. We show that Cxcl9 produced by osteoblasts interacts with vascular endothelial growth factor and prevents its binding to ECs and osteoblasts, thus abrogating angiogenesis and osteogenesis both in mouse bone and in vitro. The mechanistic target of rapamycin complex 1 activates Cxcl9 expression by transcriptional upregulation of STAT1 and increases binding of STAT1 to the Cxcl9 promoter in osteoblasts. These findings reveal the essential role of osteoblast-produced Cxcl9 in angiogenesis and osteogenesis in bone, and Cxcl9 can be targeted to elevate bone angiogenesis and prevent bone loss-related diseases.
Figures




References
-
- Magne D. et al.. Cartilage formation in growth plate and arteries: from physiology to pathology. BioEssays 27, 708–716 (2005). - PubMed
-
- Mark H., Penington A., Nannmark U., Morrison W. & Messina A. Microvascular invasion during endochondral ossification in experimental fractures in rats. Bone 35, 535–542 (2004). - PubMed
-
- Sacchetti B. et al.. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

