Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment
- PMID: 27966531
- PMCID: PMC5171850
- DOI: 10.1038/ncomms13710
Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment
Abstract
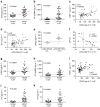
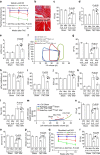
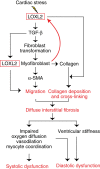
Interstitial fibrosis plays a key role in the development and progression of heart failure. Here, we show that an enzyme that crosslinks collagen-Lysyl oxidase-like 2 (Loxl2)-is essential for interstitial fibrosis and mechanical dysfunction of pathologically stressed hearts. In mice, cardiac stress activates fibroblasts to express and secrete Loxl2 into the interstitium, triggering fibrosis, systolic and diastolic dysfunction of stressed hearts. Antibody-mediated inhibition or genetic disruption of Loxl2 greatly reduces stress-induced cardiac fibrosis and chamber dilatation, improving systolic and diastolic functions. Loxl2 stimulates cardiac fibroblasts through PI3K/AKT to produce TGF-β2, promoting fibroblast-to-myofibroblast transformation; Loxl2 also acts downstream of TGF-β2 to stimulate myofibroblast migration. In diseased human hearts, LOXL2 is upregulated in cardiac interstitium; its levels correlate with collagen crosslinking and cardiac dysfunction. LOXL2 is also elevated in the serum of heart failure (HF) patients, correlating with other HF biomarkers, suggesting a conserved LOXL2-mediated mechanism of human HF.
Conflict of interest statement
C.-P.C. consults for Gilead Sciences, Inc. The remaining authors declare no competing financial interests.
Figures




References
-
- Schelbert E. B., Fonarow G. C., Bonow R. O., Butler J. & Gheorghiade M. Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J. Am. Coll. Cardiol. 63, 2188–2198 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

