Molecular mechanism: the human dopamine transporter histidine 547 regulates basal and HIV-1 Tat protein-inhibited dopamine transport
- PMID: 27966610
- PMCID: PMC5155291
- DOI: 10.1038/srep39048
Molecular mechanism: the human dopamine transporter histidine 547 regulates basal and HIV-1 Tat protein-inhibited dopamine transport
Abstract
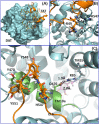
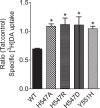
Abnormal dopaminergic transmission has been implicated as a risk determinant of HIV-1-associated neurocognitive disorders. HIV-1 Tat protein increases synaptic dopamine (DA) levels by directly inhibiting DA transporter (DAT) activity, ultimately leading to dopaminergic neuron damage. Through integrated computational modeling prediction and experimental validation, we identified that histidine547 on human DAT (hDAT) is critical for regulation of basal DA uptake and Tat-induced inhibition of DA transport. Compared to wild type hDAT (WT hDAT), mutation of histidine547 (H547A) displayed a 196% increase in DA uptake. Other substitutions of histidine547 showed that DA uptake was not altered in H547R but decreased by 99% in H547P and 60% in H547D, respectively. These mutants did not alter DAT surface expression or surface DAT binding sites. H547 mutants attenuated Tat-induced inhibition of DA transport observed in WT hDAT. H547A displays a differential sensitivity to PMA- or BIM-induced activation or inhibition of DAT function relative to WT hDAT, indicating a change in basal PKC activity in H547A. These findings demonstrate that histidine547 on hDAT plays a crucial role in stabilizing basal DA transport and Tat-DAT interaction. This study provides mechanistic insights into identifying targets on DAT for Tat binding and improving DAT-mediated dysfunction of DA transmission.
Figures




References
-
- McArthur J. C., Steiner J., Sacktor N. & Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 67, 699–714 (2010). - PubMed
-
- King J. E., Eugenin E. A., Buckner C. M. & Berman J. W. HIV tat and neurotoxicity. Microbes and infection/Institut Pasteur 8, 1347–1357 (2006). - PubMed
-
- Wang G. J. et al.. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127, 2452–2458 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

