Matrix Metalloproteinase-1 Activation Contributes to Airway Smooth Muscle Growth and Asthma Severity
- PMID: 27967204
- PMCID: PMC5422648
- DOI: 10.1164/rccm.201604-0822OC
Matrix Metalloproteinase-1 Activation Contributes to Airway Smooth Muscle Growth and Asthma Severity
Abstract
Rationale: Matrix metalloproteinase-1 (MMP-1) and mast cells are present in the airways of people with asthma.
Objectives: To investigate whether MMP-1 could be activated by mast cells and increase asthma severity.
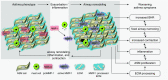
Methods: Patients with stable asthma and healthy control subjects underwent spirometry, methacholine challenge, and bronchoscopy, and their airway smooth muscle cells were grown in culture. A second asthma group and control subjects had symptom scores, spirometry, and bronchoalveolar lavage before and after rhinovirus-induced asthma exacerbations. Extracellular matrix was prepared from decellularized airway smooth muscle cultures. MMP-1 protein and activity were assessed.
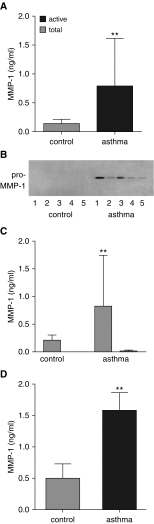
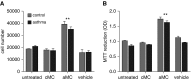
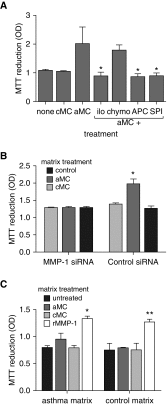
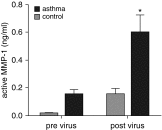
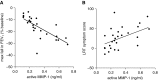
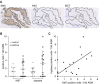
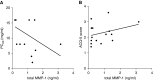
Measurements and main results: Airway smooth muscle cells generated pro-MMP-1, which was proteolytically activated by mast cell tryptase. Airway smooth muscle treated with activated mast cell supernatants produced extracellular matrix, which enhanced subsequent airway smooth muscle growth by 1.5-fold (P < 0.05), which was dependent on MMP-1 activation. In asthma, airway pro-MMP-1 was 5.4-fold higher than control subjects (P = 0.002). Mast cell numbers were associated with airway smooth muscle proliferation and MMP-1 protein associated with bronchial hyperresponsiveness. During exacerbations, MMP-1 activity increased and was associated with fall in FEV1 and worsening asthma symptoms.
Conclusions: MMP-1 is activated by mast cell tryptase resulting in a proproliferative extracellular matrix. In asthma, mast cells are associated with airway smooth muscle growth, MMP-1 levels are associated with bronchial hyperresponsiveness, and MMP-1 activation are associated with exacerbation severity. Our findings suggest that airway smooth muscle/mast cell interactions contribute to asthma severity by transiently increasing MMP activation, airway smooth muscle growth, and airway responsiveness.
Keywords: airway remodeling; airway smooth muscle; asthma; extracellular matrix; mast cells.
Figures

Comment in
-
Airway Remodeling in Asthma: Is it Fixed or Variable?Am J Respir Crit Care Med. 2017 Apr 15;195(8):968-970. doi: 10.1164/rccm.201611-2285ED. Am J Respir Crit Care Med. 2017. PMID: 28409681 No abstract available.
Similar articles
-
Expression of smooth muscle and extracellular matrix proteins in relation to airway function in asthma.J Allergy Clin Immunol. 2008 May;121(5):1196-202. doi: 10.1016/j.jaci.2008.02.017. Epub 2008 Apr 11. J Allergy Clin Immunol. 2008. PMID: 18405955
-
Extra-cellular matrix proteins induce matrix metalloproteinase-1 (MMP-1) activity and increase airway smooth muscle contraction in asthma.PLoS One. 2014 Feb 28;9(2):e90565. doi: 10.1371/journal.pone.0090565. eCollection 2014. PLoS One. 2014. PMID: 24587395 Free PMC article.
-
Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma.Am J Respir Crit Care Med. 2007 May 1;175(9):896-904. doi: 10.1164/rccm.200609-1260OC. Epub 2007 Feb 1. Am J Respir Crit Care Med. 2007. PMID: 17272787
-
Mediators on human airway smooth muscle.Clin Exp Pharmacol Physiol. 1997 Mar-Apr;24(3-4):269-72. doi: 10.1111/j.1440-1681.1997.tb01818.x. Clin Exp Pharmacol Physiol. 1997. PMID: 9131296 Review.
-
RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma.J Pharmacol Sci. 2010;114(3):239-47. doi: 10.1254/jphs.10r03cr. Epub 2010 Oct 8. J Pharmacol Sci. 2010. PMID: 20948164 Review.
Cited by
-
Crosstalk Between Signaling Pathways Involved in the Regulation of Airway Smooth Muscle Cell Hyperplasia.Front Pharmacol. 2019 Oct 9;10:1148. doi: 10.3389/fphar.2019.01148. eCollection 2019. Front Pharmacol. 2019. PMID: 31649532 Free PMC article. Review.
-
N-acetyltransferase 10 promotes the progression of oral squamous cell carcinoma through N4-acetylcytidine RNA acetylation of MMP1 mRNA.Cancer Sci. 2023 Nov;114(11):4202-4215. doi: 10.1111/cas.15946. Epub 2023 Sep 13. Cancer Sci. 2023. PMID: 37705232 Free PMC article.
-
Current Understanding of Asthma Pathogenesis and Biomarkers.Cells. 2022 Sep 5;11(17):2764. doi: 10.3390/cells11172764. Cells. 2022. PMID: 36078171 Free PMC article. Review.
-
The Cell Research Trends of Asthma: A Stem Frequency Analysis of the Literature.J Healthc Eng. 2018 Aug 23;2018:9363820. doi: 10.1155/2018/9363820. eCollection 2018. J Healthc Eng. 2018. PMID: 30210753 Free PMC article.
-
Mast cell-mediated immune regulation in health and disease.Front Med (Lausanne). 2023 Aug 17;10:1213320. doi: 10.3389/fmed.2023.1213320. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37663654 Free PMC article. Review.
References
-
- Benayoun L, Druilhe A, Dombret M-C, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. - PubMed
-
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. - PubMed
-
- Lindqvist A, Karjalainen E-M, Laitinen LA, Kava T, Altraja A, Pulkkinen M, Halme M, Laitinen A. Salmeterol resolves airway obstruction but does not possess anti-eosinophil efficacy in newly diagnosed asthma: a randomized, double-blind, parallel group biopsy study comparing the effects of salmeterol, fluticasone propionate, and disodium cromoglycate. J Allergy Clin Immunol. 2003;112:23–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

