Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway
- PMID: 27967206
- PMCID: PMC5460776
- DOI: 10.1210/en.2016-1097
Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway
Abstract
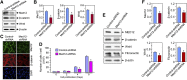
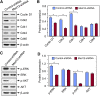
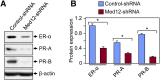
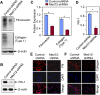
Uterine fibroids, or leiomyoma, are the most common benign tumors in women of reproductive age. In this work, the effect of silencing the mediator complex subunit 12 (Med12) gene in human uterine fibroid cells was evaluated. The role of Med12 in the modulation of Wnt/β-catenin and cell proliferation-associated signaling was evaluated in human uterine fibroid cells. Med12 was silenced in the immortalized human uterine fibroid cell line (HuLM) using a lentivirus-based Med12 gene-specific RNA interference strategy. HuLM cells were infected with lentiviruses carrying Med12-specific short hairpin RNA (shRNA) sequences or a nonfunctional shRNA scrambled control with green fluorescence protein. Stable cells that expressed low levels of Med12 protein were characterized. Wnt/β-catenin signaling, sex steroid receptor signaling, cell cycle-associated, and fibrosis-associated proteins were measured. Med12 knockdown cells showed significantly (P < 0.05) reduced levels of Wnt4 and β-catenin proteins as well as cell proliferation, as compared with scrambled control cells. Med12 knockdown cells also showed reduced levels of cell cycle-associated cyclin D1, Cdk1, and Cdk2 proteins as well as reduced activation of p-extracellular signal-regulated kinase, p-protein kinase B, and transforming growth factor (TGF)-β signaling pathways as compared with scrambled control cells. Moreover, TGF-β-regulated fibrosis-related proteins such as fibronectin, collagen type 1, and plasminogen activator inhibitor-1 were significantly (P < 0.05) reduced in Med12 knockdown cells as compared with scrambled control cells. Together, these results suggest that Med12 plays a key role in the regulation of HuLM cell proliferation through the modulation of Wnt/β-catenin, cell cycle-associated, and fibrosis-associated protein expression.
Copyright © 2017 by the Endocrine Society.
Figures

References
-
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. - PubMed
-
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33(1):59–67. - PubMed
-
- Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):615–626. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

