Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma
- PMID: 27967215
- PMCID: PMC5470749
- DOI: 10.1164/rccm.201607-1453OC
Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma
Erratum in
-
Erratum: Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma.Am J Respir Crit Care Med. 2018 Apr 1;197(7):970-971. doi: 10.1164/rccm.1977erratum1. Am J Respir Crit Care Med. 2018. PMID: 30907090 Free PMC article. No abstract available.
Abstract
Rationale: Phenotypic distinctions between severe asthma (SA) and nonsevere asthma (NONSA) may be confounded by differential adherence or incorrect use of corticosteroids.
Objectives: To determine if there are persistent phenotypic distinctions between SA (as defined by 2014 American Thoracic Society/European Respiratory Society guidelines) and NONSA after intramuscular triamcinolone acetonide (TA), and to identify predictors of a corticosteroid response in these populations.
Methods: A total of 526 adults age 18 years and older (315 SA) and 188 children age 6 to less than 18 years (107 SA) in the NHLBI Severe Asthma Research Program III were characterized before and 3 weeks after TA. The primary outcome for corticosteroid response was defined as greater than or equal to 10-point improvement in percent predicted FEV1.
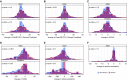
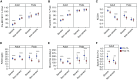
Measurements and main results: Adult asthma groups exhibited a small but significant mean FEV1% predicted improvement after TA (SA group mean difference, 3.4%; 95% confidence interval, 2.2-4.7%; P = 0.001), whereas children did not. Adult SA continued to manifest lower FEV1 and worse asthma control as compared with NONSA after TA. In children, after TA only prebronchodilator FEV1 distinguished SA from NONSA. A total of 21% of adults with SA and 20% of children with SA achieved greater than or equal to 10% improvement after TA. Baseline bronchodilator response and fractional exhaled nitric oxide had good sensitivity and specificity for predicting response in all groups except children with NONSA.
Conclusions: One in five patients with SA exhibit greater than or equal to 10% improvement in FEV1 with parenteral corticosteroid. Those likely to respond had greater bronchodilator responsiveness and fractional exhaled nitric oxide levels. In adults, differences in airflow obstruction and symptoms between SA and NONSA persist after parenteral corticosteroids, suggesting a component of corticosteroid nonresponsive pathobiology in adults with SA that may differ in children. Clinical trial registered with www.clinicaltrials.gov (NCT 01606826).
Trial registration: ClinicalTrials.gov NCT01606826.
Keywords: corticosteroid response phenotype; pediatric and adult asthma; severe asthma.
Figures

References
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. 2014;43:343–373. - PubMed
-
- European Network for Understanding Mechanisms of Severe Asthma. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. 2003;22:470–477. - PubMed
-
- Chan MT, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: clinical characteristics of steroid-insensitive asthma. 1998;101:594–601. - PubMed
-
- Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. 2007;119:405–413. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K23 HL116657/HL/NHLBI NIH HHS/United States
- U10 HL109257/HL/NHLBI NIH HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- K23 AI106945/AI/NIAID NIH HHS/United States
- K24 HL137013/HL/NHLBI NIH HHS/United States
- U10 HL109250/HL/NHLBI NIH HHS/United States
- R01 HL122531/HL/NHLBI NIH HHS/United States
- U10 HL109164/HL/NHLBI NIH HHS/United States
- U10 HL109086/HL/NHLBI NIH HHS/United States
- U10 HL109172/HL/NHLBI NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- U10 HL109168/HL/NHLBI NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- U10 HL109152/HL/NHLBI NIH HHS/United States
- U10 HL109146/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- K24 AI106822/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

