Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social-Fear Circuit
- PMID: 27974160
- PMCID: PMC5172402
- DOI: 10.1016/j.neuron.2016.10.015
Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social-Fear Circuit
Abstract
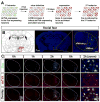
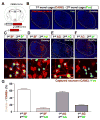
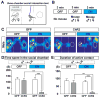
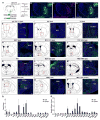
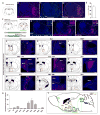
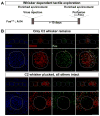
We developed a technology (capturing activated neuronal ensembles [CANE]) to label, manipulate, and transsynaptically trace neural circuits that are transiently activated in behavioral contexts with high efficiency and temporal precision. CANE consists of a knockin mouse and engineered viruses designed to specifically infect activated neurons. Using CANE, we selectively labeled neurons that were activated by either fearful or aggressive social encounters in a hypothalamic subnucleus previously known as a locus for aggression, and discovered that social-fear and aggression neurons are intermixed but largely distinct. Optogenetic stimulation of CANE-captured social-fear neurons (SFNs) is sufficient to evoke fear-like behaviors in normal social contexts, whereas silencing SFNs resulted in reduced social avoidance. CANE-based mapping of axonal projections and presynaptic inputs to SFNs further revealed a highly distributed and recurrent neural network. CANE is a broadly applicable technology for dissecting causality and connectivity of spatially intermingled but functionally distinct ensembles.
Keywords: CANE; Fos; VMHvl; activity dependent; aggression; functional neural circuit; hypothalamus; social fear.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors of this manuscript have a financial interest related to this work.
Figures



References
-
- Barnard RJ, Elleder D, Young JA. Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion. Virology. 2006;344:25–29. - PubMed
-
- Barnard RJ, Young JA. Alpharetrovirus envelope-receptor interactions. Curr Top Microbiol Immunol. 2003;281:107–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

