Nitric oxide: what's new to NO?
- PMID: 27974299
- PMCID: PMC5401944
- DOI: 10.1152/ajpcell.00315.2016
Nitric oxide: what's new to NO?
Abstract
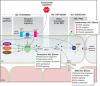
Nitric oxide (NO) is one of the critical components of the vasculature, regulating key signaling pathways in health. In macrovessels, NO functions to suppress cell inflammation as well as adhesion. In this way, it inhibits thrombosis and promotes blood flow. It also functions to limit vessel constriction and vessel wall remodeling. In microvessels and particularly capillaries, NO, along with growth factors, is important in promoting new vessel formation, a process termed angiogenesis. With age and cardiovascular disease, animal and human studies confirm that NO is dysregulated at multiple levels including decreased production, decreased tissue half-life, and decreased potency. NO has also been implicated in diseases that are related to neurotransmission and cancer although it is likely that these processes involve NO at higher concentrations and from nonvascular cell sources. Conversely, NO and drugs that directly or indirectly increase NO signaling have found clinical applications in both age-related diseases and in younger individuals. This focused review considers recently reported advances being made in the field of NO signaling regulation at several levels including enzymatic production, receptor function, interacting partners, localization of signaling, matrix-cellular and cell-to-cell cross talk, as well as the possible impact these newly described mechanisms have on health and disease.
Keywords: CD47; NOS; cardiac; cardiovascular; cytochrome b5 reductase 3; matricellular; nitric oxide; signal transduction; thrombospondin-1.
Copyright © 2017 the American Physiological Society.
Figures
References
-
- Alvira CM, Umesh A, Husted C, Ying L, Hou Y, Lyu SC, Nowak J, Cornfield DN. Voltage-dependent anion channel-2 interaction with nitric oxide synthase enhances pulmonary artery endothelial cell nitric oxide production. Am J Respir Cell Mol Biol 47: 669–678, 2012. doi:10.1165/rcmb.2011-0436OC. - DOI - PMC - PubMed
-
- Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol 58: 2683–2691, 2011. doi:10.1016/j.jacc.2011.09.033. - DOI - PMC - PubMed
-
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–339, 2002. doi:10.1038/416337a. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

