Kölliker-Fuse nuclei regulate respiratory rhythm variability via a gain-control mechanism
- PMID: 27974314
- PMCID: PMC5336570
- DOI: 10.1152/ajpregu.00238.2016
Kölliker-Fuse nuclei regulate respiratory rhythm variability via a gain-control mechanism
Abstract
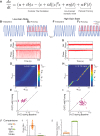
Respiration varies from breath to breath. On the millisecond timescale of spiking, neuronal circuits exhibit variability due to the stochastic properties of ion channels and synapses. Does this fast, microscopic source of variability contribute to the slower, macroscopic variability of the respiratory period? To address this question, we modeled a stochastic oscillator with forcing; then, we tested its predictions experimentally for the respiratory rhythm generated by the in situ perfused preparation during vagal nerve stimulation (VNS). Our simulations identified a relationship among the gain of the input, entrainment strength, and rhythm variability. Specifically, at high gain, the periodic input entrained the oscillator and reduced variability, whereas at low gain, the noise interacted with the input, causing events known as "phase slips", which increased variability on a slow timescale. Experimentally, the in situ preparation behaved like the low-gain model: VNS entrained respiration but exhibited phase slips that increased rhythm variability. Next, we used bilateral muscimol microinjections in discrete respiratory compartments to identify areas involved in VNS gain control. Suppression of activity in the nucleus tractus solitarii occluded both entrainment and amplification of rhythm variability by VNS, confirming that these effects were due to the activation of the Hering-Breuer reflex. Suppressing activity of the Kölliker-Fuse nuclei (KFn) enhanced entrainment and reduced rhythm variability during VNS, consistent with the predictions of the high-gain model. Together, the model and experiments suggest that the KFn regulates respiratory rhythm variability via a gain control mechanism.
Keywords: Hering-Breuer reflex; in situ preparation; respiratory rhythmogenesis; stochastic nonlinear oscillator; vagal nerve stimulation.
Copyright © 2017 the American Physiological Society.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

