Mechanisms of blood pressure salt sensitivity: new insights from mathematical modeling
- PMID: 27974315
- PMCID: PMC5407080
- DOI: 10.1152/ajpregu.00353.2016
Mechanisms of blood pressure salt sensitivity: new insights from mathematical modeling
Abstract
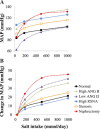
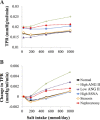
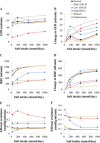
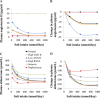
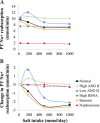
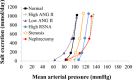
Mathematical modeling is an important tool for understanding quantitative relationships among components of complex physiological systems and for testing competing hypotheses. We used HumMod, a large physiological model, to test hypotheses of blood pressure (BP) salt sensitivity. Systemic hemodynamics, renal, and neurohormonal responses to chronic changes in salt intake were examined during normal renal function, fixed low or high plasma angiotensin II (ANG II) levels, bilateral renal artery stenosis, increased renal sympathetic nerve activity (RSNA), and decreased nephron numbers. Simulations were run for 4 wk at salt intakes ranging from 30 to 1,000 mmol/day. Reducing functional kidney mass or fixing ANG II increased salt sensitivity. Salt sensitivity, associated with inability of ANG II to respond to changes in salt intake, occurred with smaller changes in renal blood flow but greater changes in glomerular filtration rate, renal sodium reabsorption, and total peripheral resistance (TPR). However, clamping TPR at normal or high levels had no major effect on salt sensitivity. There were no clear relationships between BP salt sensitivity and renal vascular resistance or extracellular fluid volume. Our robust mathematical model of cardiovascular, renal, endocrine, and sympathetic nervous system physiology supports the hypothesis that specific types of kidney dysfunction, associated with impaired regulation of ANG II or increased tubular sodium reabsorption, contribute to BP salt sensitivity. However, increased preglomerular resistance, increased RSNA, or inability to decrease TPR does not appear to influence salt sensitivity. This model provides a platform for testing competing concepts of long-term BP control during changes in salt intake.
Keywords: angiotensin II; cardiac output; hypertension; kidney; renin-angiotensin system; salt; vascular resistance.
Copyright © 2017 the American Physiological Society.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

