16S pan-bacterial PCR can accurately identify patients with ventilator-associated pneumonia
- PMID: 27974525
- PMCID: PMC5738539
- DOI: 10.1136/thoraxjnl-2016-209065
16S pan-bacterial PCR can accurately identify patients with ventilator-associated pneumonia
Abstract
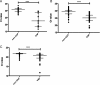
Ventilator-associated pneumonia (VAP) remains a challenge to intensive care units, with secure diagnosis relying on microbiological cultures that take up to 72 hours to provide a result. We sought to derive and validate a novel, real-time 16S rRNA gene PCR for rapid exclusion of VAP. Bronchoalveolar lavage (BAL) was obtained from two independent cohorts of patients with suspected VAP. Patients were recruited in a 2-centre derivation cohort and a 12-centre confirmation cohort. Confirmed VAP was defined as growth of >104 colony forming units/ml on semiquantitative culture and compared with a 16S PCR assay. Samples were tested from 67 patients in the derivation cohort, 10 (15%) of whom had confirmed VAP. Using cycles to cross threshold (Ct) values as the result of the 16S PCR test, the area under the receiver operating characteristic (ROC) curve (AUROC) was 0.94 (95% CI 0.86 to 1.0, p<0.0001). Samples from 92 patients were available from the confirmation cohort, 26 (28%) of whom had confirmed VAP. The AUROC for Ct in this cohort was 0.89 (95% CI 0.83 to 0.95, p<0.0001). This study has derived and assessed the diagnostic accuracy of a novel application for 16S PCR. This suggests that 16S PCR in BAL could be used as a rapid test in suspected VAP and may allow better stewardship of antibiotics.
Trial registration number: VAPRAPID trial ref NCT01972425.
Keywords: Assisted Ventilation; Bacterial Infection; Pneumonia.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: ACM is a member of the advisory board of Serendex and is chief investigator on a diagnostics study jointly funded by Innovate UK and Becton Dickinson. KT has worked on evaluations of diagnostic systems for Becton Dickinson, Cepheid, Enigma, GenMark and SelexRM has received research grant income from Innovate UK for a diagnostics consortium (with Randox Diagnostics Ltd), investigator-led grant income from Pfizer Ltd and is a consultant/advisor for Gilead Sciences Ltd. All other authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
