Thymidine Kinase-Negative Herpes Simplex Virus 1 Can Efficiently Establish Persistent Infection in Neural Tissues of Nude Mice
- PMID: 27974554
- PMCID: PMC5286872
- DOI: 10.1128/JVI.01979-16
Thymidine Kinase-Negative Herpes Simplex Virus 1 Can Efficiently Establish Persistent Infection in Neural Tissues of Nude Mice
Abstract
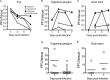
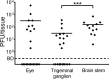
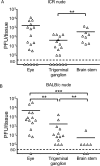
Herpes simplex virus 1 (HSV-1) establishes latency in neural tissues of immunocompetent mice but persists in both peripheral and neural tissues of lymphocyte-deficient mice. Thymidine kinase (TK) is believed to be essential for HSV-1 to persist in neural tissues of immunocompromised mice, because infectious virus of a mutant with defects in both TK and UL24 is detected only in peripheral tissues, but not in neural tissues, of severe combined immunodeficiency mice (T. Valyi-Nagy, R. M. Gesser, B. Raengsakulrach, S. L. Deshmane, B. P. Randazzo, A. J. Dillner, and N. W. Fraser, Virology 199:484-490, 1994, https://doi.org/10.1006/viro.1994.1150). Here we find infiltration of CD4 and CD8 T cells in peripheral and neural tissues of mice infected with a TK-negative mutant. We therefore investigated the significance of viral TK and host T cells for HSV-1 to persist in neural tissues using three genetically engineered mutants with defects in only TK or in both TK and UL24 and two strains of nude mice. Surprisingly, all three mutants establish persistent infection in up to 100% of brain stems and 93% of trigeminal ganglia of adult nude mice at 28 days postinfection, as measured by the recovery of infectious virus. Thus, in mouse neural tissues, host T cells block persistent HSV-1 infection, and viral TK is dispensable for the virus to establish persistent infection. Furthermore, we found 30- to 200-fold more virus in neural tissues than in the eye and detected glycoprotein C, a true late viral antigen, in brainstem neurons of nude mice persistently infected with the TK-negative mutant, suggesting that adult mouse neurons can support the replication of TK-negative HSV-1.
Importance: Acyclovir is used to treat herpes simplex virus 1 (HSV-1)-infected immunocompromised patients, but treatment is hindered by the emergence of drug-resistant viruses, mostly those with mutations in viral thymidine kinase (TK), which activates acyclovir. TK mutants are detected in brains of immunocompromised patients with persistent infection. However, answers to the questions as to whether TK-negative (TK-) HSV-1 can establish persistent infection in brains of immunocompromised hosts and whether neurons in vivo are permissive for TK- HSV-1 remain elusive. Using three genetically engineered HSV-1 TK- mutants and two strains of nude mice deficient in T cells, we found that all three HSV-1 TK- mutants can efficiently establish persistent infection in the brain stem and trigeminal ganglion and detected glycoprotein C, a true late viral antigen, in brainstem neurons. Our study provides evidence that TK- HSV-1 can persist in neural tissues and replicate in brain neurons of immunocompromised hosts.
Keywords: herpes simplex virus 1; mice; neural tissues; persistent infection; thymidine kinase.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Diverse herpes simplex virus type 1 thymidine kinase mutants in individual human neurons and Ganglia.J Virol. 2007 Jul;81(13):6817-26. doi: 10.1128/JVI.00166-07. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459924 Free PMC article.
-
Importance of the herpes simplex virus UL24 gene for productive ganglionic infection in mice.Virology. 1998 Mar 1;242(1):161-9. doi: 10.1006/viro.1997.9012. Virology. 1998. PMID: 9501052
-
A thymidine kinase-negative HSV-1 strain establishes a persistent infection in SCID mice that features uncontrolled peripheral replication but only marginal nervous system involvement.Virology. 1994 Mar;199(2):484-90. doi: 10.1006/viro.1994.1150. Virology. 1994. PMID: 8122378
-
Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management.Antimicrob Agents Chemother. 2011 Feb;55(2):459-72. doi: 10.1128/AAC.00615-10. Epub 2010 Nov 15. Antimicrob Agents Chemother. 2011. PMID: 21078929 Free PMC article. Review.
-
Control of HSV-1 latency in human trigeminal ganglia--current overview.J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
-
Analysis of MicroRNA Expression in Tears of Patients with Herpes Epithelial Keratitis: A Preliminary Study.Invest Ophthalmol Vis Sci. 2022 Apr 1;63(4):21. doi: 10.1167/iovs.63.4.21. Invest Ophthalmol Vis Sci. 2022. PMID: 35475887 Free PMC article.
-
Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets.Front Microbiol. 2019 May 8;10:941. doi: 10.3389/fmicb.2019.00941. eCollection 2019. Front Microbiol. 2019. PMID: 31134006 Free PMC article. Review.
-
Targeting m6A modification inhibits herpes virus 1 infection.Genes Dis. 2021 Feb 22;9(4):1114-1128. doi: 10.1016/j.gendis.2021.02.004. eCollection 2022 Jul. Genes Dis. 2021. PMID: 35685469 Free PMC article.
-
Suppression of annexin A1 and its receptor reduces herpes simplex virus 1 lethality in mice.PLoS Pathog. 2022 Aug 8;18(8):e1010692. doi: 10.1371/journal.ppat.1010692. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35939498 Free PMC article.
-
"Non-Essential" Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review.Viruses. 2020 Dec 23;13(1):17. doi: 10.3390/v13010017. Viruses. 2020. PMID: 33374862 Free PMC article. Review.
References
-
- Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Stranska R, van Loon AM, Polman M, Beersma MF, Bredius RG, Lankester AC, Meijer E, Schuurman R. 2004. Genotypic and phenotypic characterization of acyclovir-resistant herpes simplex viruses isolated from haematopoietic stem cell transplant recipients. Antivir Ther 9:565–575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

