Rapid synthesis of cyclic oligomeric depsipeptides with positional, stereochemical, and macrocycle size distribution control
- PMID: 27974608
- PMCID: PMC5206571
- DOI: 10.1073/pnas.1616462114
Rapid synthesis of cyclic oligomeric depsipeptides with positional, stereochemical, and macrocycle size distribution control
Erratum in
-
Correction for Batiste and Johnston, Rapid synthesis of cyclic oligomeric depsipeptides with positional, stereochemical, and macrocycle size distribution control.Proc Natl Acad Sci U S A. 2021 Jul 6;118(27):e2110836118. doi: 10.1073/pnas.2110836118. Proc Natl Acad Sci U S A. 2021. PMID: 34183425 Free PMC article. No abstract available.
Abstract
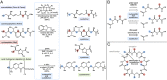
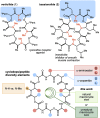
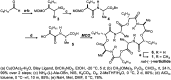
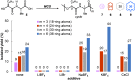
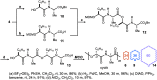
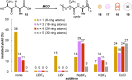
Macrocyclic small molecules are attractive tools in the development of sensors, new materials, and therapeutics. Within early-stage drug discovery, they are increasingly sought for their potential to interact with broad surfaces of peptidic receptors rather than within their narrow folds and pockets. Cyclization of linear small molecule precursors is a straightforward strategy to constrain conformationally mobile motifs, but forging a macrocycle bond typically becomes more difficult at larger ring sizes. We report the development of a general approach to discrete collections of oligomeric macrocyclic depsipeptides using an oligomerization/macrocyclization process governed by a series of Mitsunobu reactions of hydroxy acid monomers. Ring sizes of 18, 24, 30, and 36 are formed in a single reaction from a didepsipeptide, whereas sizes of 24, 36, and 60 result from a tetradepsipeptide. The ring-size selectivity inherent to the approach can be modulated by salt additives that enhance the formation of specific ring sizes. Use of chemical synthesis to prepare the monomers suggests broad access to functionally and stereochemically diverse collections of natural product-like oligodepsipeptide macrocycles. Two cyclodepsipeptide natural products were prepared along with numerous unnatural oligomeric congeners to provide rapid access to discrete collections of complex macrocyclic small molecules from medium (18) to large (60) ring sizes.
Keywords: Mitsunobu; collective; macrocycle; oligomerization; total synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

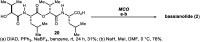
References
-
- Woodward RB, et al. Asymmetric total synthesis of erythromycin. 2. Synthesis of an erythronolide A lactone system. J Am Chem Soc. 1981;103(11):3213–3215.
-
- Paterson I, Laffan DDP, Rawson DJ. Studies in macrolide synthesis: A concise asymmetric synthesis of a macrolide intermediate for the erythronolides. Tetrahedron Lett. 1988;29(12):1461–1464.
-
- Evans DA, Dinsmore CJ, Evrard DA, DeVries KM. Oxidative coupling of arylglycine-containing peptides. A biomimetic approach to the synthesis of the macrocyclic actinoidinic-containing vancomycin subunit. J Am Chem Soc. 1993;115(14):6426–6427.
-
- Boger DL, Nomoto Y, Teegarden BR. Vancomycin and ristocetin models: Synthesis via the Ullmann macrocyclization reaction. J Org Chem. 1993;58(6):1425–1433.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

