Activation of Glutamatergic Fibers in the Anterior NAc Shell Modulates Reward Activity in the aNAcSh, the Lateral Hypothalamus, and Medial Prefrontal Cortex and Transiently Stops Feeding
- PMID: 27974611
- PMCID: PMC6705665
- DOI: 10.1523/JNEUROSCI.1605-16.2016
Activation of Glutamatergic Fibers in the Anterior NAc Shell Modulates Reward Activity in the aNAcSh, the Lateral Hypothalamus, and Medial Prefrontal Cortex and Transiently Stops Feeding
Abstract
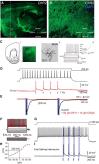
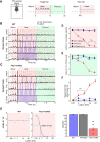
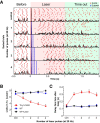
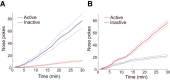
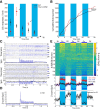
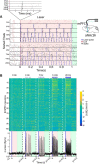
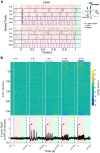
Although the release of mesoaccumbal dopamine is certainly involved in rewarding responses, recent studies point to the importance of the interaction between it and glutamate. One important component of this network is the anterior nucleus accumbens shell (aNAcSh), which sends GABAergic projections into the lateral hypothalamus (LH) and receives extensive glutamatergic inputs from, among others, the medial prefrontal cortex (mPFC). The effects of glutamatergic activation of aNAcSh on the ingestion of rewarding stimuli as well as its effect in the LH and mPFC are not well understood. Therefore, we studied behaving mice that express a light-gated channel (ChR2) in glutamatergic fibers in their aNAcSh while recording from neurons in the aNAcSh, or mPFC or LH. In Thy1-ChR2, but not wild-type, mice activation of aNAcSh fibers transiently stopped the mice licking for sucrose or an empty sipper. Stimulation of aNAcSh fibers both activated and inhibited single-unit responses aNAcSh, mPFC, and LH, in a manner that maintains firing rate homeostasis. One population of licking-inhibited pMSNs in the aNAcSh was also activated by optical stimulation, suggesting their relevance in the cessation of feeding. A rewarding aspect of stimulation of glutamatergic inputs was found when the Thy1-ChR2 mice learned to nose-poke to self-stimulate these inputs, indicating that bulky stimulation of these fibers are rewarding in the sense of wanting. Stimulation of excitatory afferents evoked both monosynaptic and polysynaptic responses distributed in the three recorded areas. In summary, we found that activation of glutamatergic aNAcSh fibers is both rewarding and transiently inhibits feeding.
Significance statement: We have established that the activation of glutamatergic fibers in the anterior nucleus accumbens shell (aNAcSh) transiently stops feeding and yet, because mice self-stimulate, is rewarding in the sense of wanting. Moreover, we have characterized single-unit responses of distributed components of a hedonic network (comprising the aNAcSh, medial prefrontal cortex, and lateral hypothalamus) recruited by activation of glutamatergic aNAcSh afferents that are involved in encoding a positive valence signal important for the wanting of a reward and that transiently stops ongoing consummatory actions, such as licking.
Keywords: Thy1-ChR2; feeding; glutamatergic afferents; licking; optogenetics; reward.
Copyright © 2016 the authors 0270-6474/16/3612511-19$15.00/0.
Figures




Similar articles
-
Role of dopamine projections from ventral tegmental area to nucleus accumbens and medial prefrontal cortex in reinforcement behaviors assessed using optogenetic manipulation.Metab Brain Dis. 2017 Oct;32(5):1491-1502. doi: 10.1007/s11011-017-0023-3. Epub 2017 May 19. Metab Brain Dis. 2017. PMID: 28523568
-
Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking.Nature. 2011 Jun 29;475(7356):377-80. doi: 10.1038/nature10194. Nature. 2011. PMID: 21716290 Free PMC article.
-
Feeding and Reward Are Differentially Induced by Activating GABAergic Lateral Hypothalamic Projections to VTA.J Neurosci. 2016 Mar 9;36(10):2975-85. doi: 10.1523/JNEUROSCI.3799-15.2016. J Neurosci. 2016. PMID: 26961951 Free PMC article.
-
Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward.Crit Rev Neurobiol. 2000;14(2):131-42. Crit Rev Neurobiol. 2000. PMID: 11513242 Review.
-
Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens.Psychopharmacology (Berl). 2017 May;234(9-10):1439-1449. doi: 10.1007/s00213-016-4522-4. Epub 2017 Jan 4. Psychopharmacology (Berl). 2017. PMID: 28054099 Free PMC article. Review.
Cited by
-
A hippocampus-accumbens code guides goal-directed appetitive behavior.Nat Commun. 2024 Apr 12;15(1):3196. doi: 10.1038/s41467-024-47361-x. Nat Commun. 2024. PMID: 38609363 Free PMC article.
-
Optical Intracranial Self-Stimulation (oICSS): A New Behavioral Model for Studying Drug Reward and Aversion in Rodents.Int J Mol Sci. 2024 Mar 19;25(6):3455. doi: 10.3390/ijms25063455. Int J Mol Sci. 2024. PMID: 38542425 Free PMC article. Review.
-
Reward-representing D1-type neurons in the medial shell of the accumbens nucleus regulate palatable food intake.Int J Obes (Lond). 2019 Apr;43(4):917-927. doi: 10.1038/s41366-018-0133-y. Epub 2018 Jun 15. Int J Obes (Lond). 2019. PMID: 29907842 Free PMC article.
-
Optoception: Perception of Optogenetic Brain Perturbations.eNeuro. 2022 Jun 28;9(3):ENEURO.0216-22.2022. doi: 10.1523/ENEURO.0216-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35715209 Free PMC article.
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
