Early dissemination seeds metastasis in breast cancer
- PMID: 27974799
- PMCID: PMC5390864
- DOI: 10.1038/nature20785
Early dissemination seeds metastasis in breast cancer
Abstract
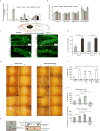
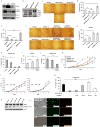
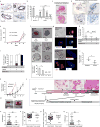
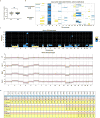
Accumulating data suggest that metastatic dissemination often occurs early during tumour formation, but the mechanisms of early metastatic spread have not yet been addressed. Here, by studying metastasis in a HER2-driven mouse breast cancer model, we show that progesterone-induced signalling triggers migration of cancer cells from early lesions shortly after HER2 activation, but promotes proliferation in advanced primary tumour cells. The switch from migration to proliferation was regulated by increased HER2 expression and tumour-cell density involving microRNA-mediated progesterone receptor downregulation, and was reversible. Cells from early, low-density lesions displayed more stemness features, migrated more and founded more metastases than cells from dense, advanced tumours. Notably, we found that at least 80% of metastases were derived from early disseminated cancer cells. Karyotypic and phenotypic analysis of human disseminated cancer cells and primary tumours corroborated the relevance of these findings for human metastatic dissemination.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





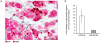
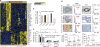
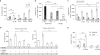

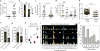

Comment in
-
Timing of Metastasis in Breast Cancer.N Engl J Med. 2017 Jun 22;376(25):2486-2488. doi: 10.1056/NEJMcibr1701388. N Engl J Med. 2017. PMID: 28636861 No abstract available.
References
-
- Cole BF, Gelber RD, Gelber S, Coates AS, Goldhirsch A. Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet. 2001;358:277–286. - PubMed
-
- Gianni L, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-XS1470-2045(11)70033-X [pii] - PubMed
-
- Klein CA, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002;360:683–689. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

