Human dental stem cells suppress PMN activity after infection with the periodontopathogens Prevotella intermedia and Tannerella forsythia
- PMID: 27974831
- PMCID: PMC5156907
- DOI: 10.1038/srep39096
Human dental stem cells suppress PMN activity after infection with the periodontopathogens Prevotella intermedia and Tannerella forsythia
Abstract
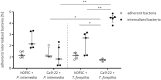
Periodontitis is characterized by inflammation associated with the colonization of different oral pathogens. We here aimed to investigate how bacteria and host cells shape their environment in order to limit inflammation and tissue damage in the presence of the pathogen. Human dental follicle stem cells (hDFSCs) were co-cultured with gram-negative P. intermedia and T. forsythia and were quantified for adherence and internalization as well as migration and interleukin secretion. To delineate hDFSC-specific effects, gingival epithelial cells (Ca9-22) were used as controls. Direct effects of hDFSCs on neutrophils (PMN) after interaction with bacteria were analyzed via chemotactic attraction, phagocytic activity and NET formation. We show that P. intermedia and T. forsythia adhere to and internalize into hDFSCs. This infection decreased the migratory capacity of the hDFSCs by 50%, did not disturb hDFSC differentiation potential and provoked an increase in IL-6 and IL-8 secretion while leaving IL-10 levels unaltered. These environmental modulations correlated with reduced PMN chemotaxis, phagocytic activity and NET formation. Our results suggest that P. intermedia and T. forsythia infected hDFSCs maintain their stem cell functionality, reduce PMN-induced tissue and bone degradation via suppression of PMN-activity, and at the same time allow for the survival of the oral pathogens.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

