Sialylation of N-glycans: mechanism, cellular compartmentalization and function
- PMID: 27975143
- PMCID: PMC7088086
- DOI: 10.1007/s00418-016-1520-x
Sialylation of N-glycans: mechanism, cellular compartmentalization and function
Abstract
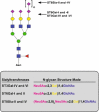
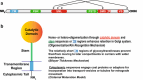
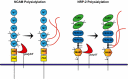
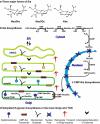
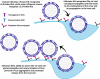
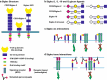
Sialylated N-glycans play essential roles in the immune system, pathogen recognition and cancer. This review approaches the sialylation of N-glycans from three perspectives. The first section focuses on the sialyltransferases that add sialic acid to N-glycans. Included in the discussion is a description of these enzymes' glycan acceptors, conserved domain organization and sequences, molecular structure and catalytic mechanism. In addition, we discuss the protein interactions underlying the polysialylation of a select group of adhesion and signaling molecules. In the second section, the biosynthesis of sialic acid, CMP-sialic acid and sialylated N-glycans is discussed, with a special emphasis on the compartmentalization of these processes in the mammalian cell. The sequences and mechanisms maintaining the sialyltransferases and other glycosylation enzymes in the Golgi are also reviewed. In the final section, we have chosen to discuss processes in which sialylated glycans, both N- and O-linked, play a role. The first part of this section focuses on sialic acid-binding proteins including viral hemagglutinins, Siglecs and selectins. In the second half of this section, we comment on the role of sialylated N-glycans in cancer, including the roles of β1-integrin and Fas receptor N-glycan sialylation in cancer cell survival and drug resistance, and the role of these sialylated proteins and polysialic acid in cancer metastasis.
Keywords: Golgi; Polysialic acid; Selectins; Sialic acid; Sialyltransferase; Siglecs.
Figures

References
-
- Angata K, Suzuki M, McAuliffe J, Ding Y, Hindsgaul O, Fukuda M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct a2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J Biol Chem. 2000;275:18594–18601. doi: 10.1074/jbc.M910204199. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

