Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants
- PMID: 27976361
- PMCID: PMC6463790
- DOI: 10.1002/14651858.CD005384.pub2
Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants
Update in
-
Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants.Cochrane Database Syst Rev. 2023 Jul 19;7(7):CD005384. doi: 10.1002/14651858.CD005384.pub3. Cochrane Database Syst Rev. 2023. PMID: 37466143 Free PMC article. Review.
Abstract
Background: Nasal continuous positive airway pressure (NCPAP) is a strategy for maintaining positive airway pressure throughout the respiratory cycle through the application of bias flow of respiratory gas to an apparatus attached to the nose. Treatment with NCPAP is associated with decreased risk of mechanical ventilation and might be effective in reducing chronic lung disease. Nasal intermittent positive pressure ventilation (NIPPV) is a form of noninvasive ventilation during which patients are exposed intermittently to higher levels of airway pressure, along with NCPAP through the same nasal device.
Objectives: To examine the risks and benefits of early NIPPV versus early NCPAP alone for preterm infants at risk of or in respiratory distress within the first hours after birth.Primary endpoints are respiratory failure and the need for intubated ventilatory support during the first week of life. Secondary endpoints include chronic lung disease (CLD) (oxygen therapy at 36 weeks' postmenstrual age), air leaks, duration of respiratory support, duration of oxygen therapy, intraventricular hemorrhage, and incidence of mortality.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9), MEDLINE via PubMed (1966 to September 28, 2015), Embase (1980 to September 28, 2015), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to September 28, 2015). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi-randomized trials. A member of the Cochrane Neonatal Review Group handsearched abstracts from the European Society of Pediatric Research (ESPR). We contacted the authors of ongoing clinical trials to ask for information.
Selection criteria: We considered all randomized and quasi-randomized controlled trials. Studies selected compared NIPPV versus NCPAP treatment, starting at birth or shortly thereafter in preterm infants (< 37 weeks' gestational age).
Data collection and analysis: We performed data collection and analysis using the recommendations of the Cochrane Neonatal Review Group.
Main results: Ten trials, enrolling a total of 1061 infants, met criteria for inclusion in this review. Meta-analyses of these studies showed significantly reduced risk of meeting respiratory failure criteria (typical risk ratio (RR) 0.65, 95% confidence interval (CI) 0.51 to 0.82; typical risk difference (RD) -0.09, 95% CI -0.13 to -0.04) and needing intubation (typical RR 0.78, 95% CI 0.64 to 0.94; typical RD -0.07, 95% CI -0.12 to -0.02) among infants treated with early NIPPV compared with early NCPAP. The meta-analysis did not demonstrate a reduction in the risk of CLD among infants randomized to NIPPV (typical RR 0.78, 95% CI 0.58 to 1.06). Investigators observed no evidence of harm. Review authors graded the quality of the evidence as moderate (unblinded studies).
Authors' conclusions: Early NIPPV does appear to be superior to NCPAP alone for decreasing respiratory failure and the need for intubation and endotracheal tube ventilation among preterm infants with respiratory distress syndrome. Additional studies are needed to confirm these results and to assess the safety of NIPPV compared with NCPAP alone in a larger patient population.
Conflict of interest statement
Review authors acknowledge no implied or actual potential conflict of interest.
Figures
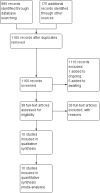







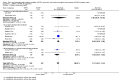

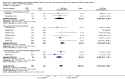






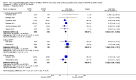



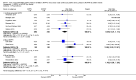








References
References to studies included in this review
Armanian 2014 {published data only}
-
- Armanian AM, Badiee Z, Heidari G, Feizi A, Salehimehr N. Initial treatment of respiratory distress syndrome with nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure: a randomized controlled trial. International Journal of Preventive Medicine 2014;5(12):1543‐51. [PUBMED: 25709790] - PMC - PubMed
Bisceglia 2007 {published and unpublished data}
-
- Bisceglia M, Belcastro A, Poerio V, Raimondi F, Mesuraca L, Crugliano C, et al. A comparison of nasal intermittent versus continuous positive pressure delivery for the treatment of moderate respiratory distress syndrome in preterm infants. Minerva Pediatrica 2007;59(2):91‐5. - PubMed
Kirpalani 2013 {unpublished data only}
-
- Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS. NIPPV Study Group. A trial comparing noninvasive ventilation strategies in preterm infants. New England Journal of Medicine 2013;369(7):611‐20. - PubMed
Kugelman 2007 {published data only}
-
- Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized, controlled, prospective study. Journal of Pediatrics 2007;150(5):521‐6. - PubMed
Lista 2009 {published data only}
-
- Lista G, Castoldi F, Fontana P, Daniele I, Cavigioli F, Rossi S, et al. Nasal continuous positive airway pressure (CPAP) versus bi‐level nasal CPAP in preterm babies with respiratory distress syndrome: a randomised control trial. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;95(2):F85‐9. - PubMed
Meneses 2011 {published and unpublished data}
-
- Meneses J, Bhandari V, Alves JG, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: a randomized controlled trial. Pediatrics 2011;127(2):300‐7. - PubMed
Ramanathan 2012 {published data only}
-
- Ramanathan R, Sekar KC, Rasmussen M, Bathia J, Soll RF. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants under 30 weeks gestation: a randomized controlled trial. Journal of Perinatology 2012;32(5):336‐43. - PubMed
Sai Sunil Kishore 2009 {published data only}
-
- Sai Sunil Kishore M, Dutta S, Kumar P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatrica 2009;98(9):1412‐5. - PubMed
Salama 2015 {published data only}
-
- Salama GS, Ayyash FF, Al‐Rabadi AJ, Alquran ML, Shakkoury AG. Nasal‐IMV versus nasal‐CPAP as an initial mode of respiratory support for premature infants with RDS: a prospective randomized clinical trial. Rawal Journal Medical 2015;40(2):197‐202.
Wood 2013 {published data only}
-
- Wood FE, Gupta S, Tin W, Sinha S. Randomised controlled trial of synchronised intermittent positive airway pressure (SiPAP) versus continuous positive airway pressure (CPAP) as a primary mode of respiratory support in preterm infants with respiratory distress syndrome. Archives of Disease in Childhood 2013;98(Suppl 1):A1‐117.
References to studies excluded from this review
Aghai 2006 {published data only}
-
- Aghai ZH, Saslow JG, Nakhla T, Milcarek BB, Hart J, Lawrysh‐Plunkett R, et al. Synchronized nasal intermittent positive pressure ventilation (SNIPPV) decreases work of breathing (WOB) in premature infants with respiratory distress syndrome (RDS) compared to nasal continuous positive airway pressure (NCPAP). Pediatric Pulmonology 2006;41(9):875‐81. - PubMed
Baneshi 2014 {published data only}
-
- Baneshi MR, Bahmanbijari B, Mahdian R, Haji‐Maghsoodi S, Nikbakht R. Comparison of nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure treatments using parametric survival models. Iranian Journal of Pediatrics 2014;24(2):207‐13. [PUBMED: 25535541] - PMC - PubMed
Barrington 2001 {published data only}
-
- Barrington KJ, Bull D, Finer NN. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics 2001;107(4):638‐41. [PUBMED: 11335736] - PubMed
Bhandari 2007 {published data only}
-
- Bhandari V, Gavino RG, Nedrelow JH, Pallela P, Salvador A, Ehrenkranz RA, et al. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. Journal of Perinatology 2007;27(11):697‐703. - PubMed
Chen 2015 {published data only}
Friedlich 1999 {published data only}
-
- Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal‐synchronised intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants following extubation. Journal of Perinatology 1999;19(6 Pt 1):413‐8. - PubMed
Gao 2010 {published data only}
-
- Gao WW, Tan SZ, Chen YB, Zhang Y, Wang Y. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2010;12(7):524‐6. [PUBMED: 20637147] - PubMed
Gizzi 2015 {published data only}
-
- Gizzi C, Montecchia F, Panetta V, Castellano C, Mariani C, Campelli M, et al. Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross‐over trial. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2015;100(1):F17‐23. [PUBMED: 25318667] - PubMed
Herber‐Jonat 2006 {published data only}
-
- Herber‐Jonat S, Rieger‐Fackeldey E, Hummler H, Schulze A. Adaptive mechanical backup ventilation for preterm infants on respiratory assist modes ‐ a pilot study. Intensive Care Medicine 2006;32(2):302‐8. - PubMed
Jasani 2016 {published data only}
-
- Jasani B, Nanavati R, Kabra N, Rajdeo S, Bhandari V. Comparison of non‐synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as post‐extubation respiratory support in preterm infants with respiratory distress syndrome: a randomized controlled trial. Journal of Maternal and Fetal Medicine 2016;29(10):1546‐51. - PubMed
Kahramaner 2014 {published data only}
-
- Kahramaner Z, Erdemir A, Turkoglu E, Cosar H, Sutcuoglu S, Ozer EA. Unsynchronized nasal intermittent positive pressure versus nasal continuous positive airway pressure in preterm infants after extubation. Journal of Maternal and Fetal Neonatal Medicine 2014;27(9):926‐9. - PubMed
Khalaf 2001 {published data only}
-
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomised controlled trial comparing synchronized nasal intermittent positive pressure ventilation (SNIPPV) versus nasal continuous positive airway pressure (NCPAP) as mode of extubation. Pediatric Research 1999;45:204a. - PubMed
-
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics 2001;108(1):13‐7. [PUBMED: 11433048] - PubMed
Khorana 2008 {published data only}
-
- Khorana M, Paradeevisut H, Sangtawesin V, Kanjanapatanakul W, Chotigeat U, Ayutthaya JK. A randomized trial of non‐synchronized nasopharyngeal intermittent mandatory ventilation (nsNIMV) vs. nasal continuous positive airway pressure (nCPAP) in the prevention of extubation failure in preterm under 1500 grams. Journal of the Medical Association of Thailand 2008;91(3):S136‐42. - PubMed
Kugelman 2014a {published data only}
-
- Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D. A randomized pilot study comparing heated humidified high‐flow nasal cannulae with NIPPV for RDS. Pediatric Pulmonology 2015;50(6):576‐83. [PUBMED: 24619945] - PubMed
Lin 1998 {published data only}
-
- Lin CH, Wang ST, Lin YJ, Yeh TF. Efficacy of nasal intermittent positive pressure ventilation in treating apnea of prematurity. Pediatric Pulmonology 1998;26(5):349‐53. - PubMed
Lin 2011 {published data only}
-
- Lin XZ, Zheng Z, Lin YY, Lai JD, Li YD. [Nasal synchronized intermittent positive pressure ventilation for the treatment of apnea in preterm infants]. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2011;13(10):783‐6. [PUBMED: 22000430] - PubMed
Liu 2003 {published data only}
-
- Liu T, Tong F, Du LZ, Shi LP. Clinical observation of variable‐flow nasal continuous positive airway pressure in preterm neonates with respiratory failure. Zhonghua Er Ke Za Zhi (Chinese Journal of Pediatrics) 2003;41(6):473‐4. - PubMed
Manzar 2004 {published data only}
-
- Manzar S, Nair AK, Pai MG, Paul J, Manikoth P, Georage M, et al. Use of nasal intermittent positive pressure ventilation to avoid intubation in neonates. Saudi Medical Journal 2004;25(10):1464‐7. - PubMed
Migliori 2005 {published data only}
-
- Migliori C, Motta M, Angeli A, Chirico G. Nasal bilevel vs. continuous positive airway pressure in preterm infants. Pediatric Pulmonology 2005;40(5):426‐30. - PubMed
Moretti 2008 {published data only}
-
- Moretti C, Giannini L, Fassi C, Gizzi C, Papoff P, Colarizi P. Nasal flow‐synchronized intermittent positive pressure ventilation to facilitate weaning in very low‐birthweight infants: unmasked randomized controlled trial. Pediatrics International 2008;50(1):85‐91. [PUBMED: 18279212] - PubMed
O'Brien 2012 {published data only}
Pantalitschka 2009 {published data only}
-
- Pantalitschka T, Sievers J, Urschitz MS, Herberts T, Reher C, Poets CF. Randomised crossover trial of four nasal respiratory support systems for apnoea of prematurity in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94(4):F245‐8. [PUBMED: 19131432] - PubMed
Ramanathan 2009 {unpublished data only}
-
- Ramanathan R, Sekar K, Rasmussen M, Bhatia J, Soll R. Nasal intermittent positive pressure ventilation (NIPPV) versus synchronized intermittent mandatory ventilation (SIMV) after surfactant treatment for respiratory distress syndrome (RDS) in preterm infants <30 weeks' gestation: multicenter, randomized, clinical trial. Pediatric Academic Society Annual Meeting. 2009; Vol. Oral Presentation:3212.6.
Ryan 1989 {published data only}
-
- Ryan CA, Finer NN, Peters KL. Nasal intermittent positive‐pressure ventilation offers no advantages over nasal continuous positive airway pressure in apnea of prematurity. American Journal of Diseases of Children 1989;143(10):1196‐8. - PubMed
Salvo 2015 {published data only}
-
- Salvo V, Lista G, Lupo E, Ricotti A, Zimmermann LJ, Gavilanes AW, et al. Noninvasive ventilation strategies for early treatment of RDS in preterm infants: an RCT. Pediatrics 2015;135(3):444‐51. - PubMed
Santin 2004 {published data only}
-
- Santin R, Brodsky N, Bhandari V. A prospective observational pilot study of synchronized nasal intermittent positive pressure ventilation (SNIPPV) as a primary mode of ventilation in infants ≥ 28 weeks with respiratory distress syndrome (RDS). Journal of Perinatology 2004;24(8):487‐93. - PubMed
Shi 2010 {published data only}
-
- Shi Y, Tang S, Zhao J, Hu Z, Li T. Efficiency of nasal intermittent positive pressure ventilation vs nasal continuous positive airway pressure on neonatal respiratory distress syndrome: a prospective, randomized, controlled study. Acta Academiae Medicinae Militaris Tertiae 2010;32(18):1991‐3.
Shi 2014 {published data only}
-
- Shi Y, Tang S, Zhao J, Shen J. A prospective, randomized, controlled study of NIPPV versus nCPAP in preterm and term infants with respiratory distress syndrome. Pediatric Pulmonology 2014;49(7):673‐8. - PubMed
Zhou 2015 {published data only}
-
- Zhou B, Zhai JF, Jiang HX, Liu Y, Jin B, Zhang YY, et al. Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome. European Review for Medical and Pharmacological Sciences 2015;19(4):573‐7. [PUBMED: 25753873] - PubMed
References to studies awaiting assessment
Chen 2013 {published data only}
-
- Chen X, Peng WS, Wang L, Xu JL, Dong HF, Pan JH. [A randomized controlled study of nasal intermittent positive pressure ventilation in the treatment of neonatal respiratory distress syndrome].. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2013;15(9):713‐7. - PubMed
Fu 2014 {published data only}
-
- Fu CH, Xia SW. Clinical application of nasal intermittent positive pressure ventilation in initial treatment of neonatal respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2014;16(5):460‐4. [PUBMED: 24856992] - PubMed
Gao 2014 {published data only}
-
- Gao X, Yang B, Hei M, Cui X, Wang J, Zhou G, et al. Application of three kinds of non‐invasive positive pressure ventilation as a primary mode of ventilation in premature infants with respiratory distress syndrome: a randomized controlled trial. Zhonghua Dang Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2014;52(1):34‐40. [PUBMED: 24680406] - PubMed
Kong 2012 {published data only}
-
- Kong LK, Kong XY, Li LH, Dong JY, Shang MX, Chi JH, et al. Comparative study on application of Duo positive airway pressure and continuous positive airway pressure in preterm neonates with respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2012;14(12):888‐92. [PUBMED: 23234771] - PubMed
Sasi 2013 {published data only}
-
- Sasi A, Skariah T, Lewis L. Early nasal intermittent mandatory ventilation (NIMV) versus nasal continuous positive airway pressure (NCPAP) for respiratory distress syndrome (RDS) in infants 28 to 36 weeks gestation ‐ a randomized controlled trial. Journal of Paediatrics and Child Health 2013;49(Suppl 2):34‐5.
Silveira 2015 {published data only}
-
- Silveira CS, Leonardi KM, Melo AP, Zaia JE, Brunherotti MA. Response of preterm infants to 2 noninvasive ventilatory support systems: nasal CPAP and nasal intermittent positive‐pressure ventilation. Respiratory Care 2015;60(12):1772‐6. [PUBMED: 26374907] - PubMed
References to ongoing studies
Sabzehei 2015 {published data only}
-
- Early nasal intermittent positive pressure ventilation vs continuous positive airway pressure in preterm infants with respiratory distress syndrome. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Avery 1987
-
- Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79(1):26‐30. - PubMed
Bancalari 2001
-
- Bancalari E, Claure N. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. American Journal of Perinatology 2001;18(1):1‐9. - PubMed
Bell 1978
Cappelleri 1996
-
- Cappelleri JC, Ioannidis JP, Schmid CH, Ferranti SD, Aubert M, Chalmers TC, et al. Large trials vs meta‐analysis of smaller trials: how do their results compare?. Journal of the American Medical Association 1996;276(16):1332‐8. - PubMed
Finer 2004
-
- Finer NN, Carlo WA, Duara S, Fanaroff AA, Donovan EF, Wright LL, et al. Delivery room continuous positive airway pressure/positive end‐expiratory pressure in extremely low birth weight infants: a feasibility trial. Pediatrics 2004;114(3):651‐7. - PubMed
Garland 1985
-
- Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 1985;76(3):406‐10. - PubMed
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
ICCROP 2005
-
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. - PubMed
Kiciman 1998
-
- Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatric Pulmonology 1998;25(3):175‐81. - PubMed
Lemyre 2002
Lemyre 2014
Meyer 2004
-
- Meyer M, Mildenhall L, Wong M. Outcomes for infants weighing less than 1000 grams cared for with a nasal continuous positive airway pressure‐based strategy. Journal of Paediatrcs and Child Health 2004;40(1):38‐41. - PubMed
Moretti 1999
-
- Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, et al. Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Human Development 1999;56(2‐3):166‐77. - PubMed
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook Updated October 2013.
Stoelhorst 2005
-
- Stoelhorst GM, Rijken M, Martens SE, Brand R, Ouden AL, Wit JM, et al. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the Project On Preterm and Small for Gestational Age Infants 1983 and the Leiden Follow‐Up Project on Prematurity 1996‐1997. Pediatrics 2005;115(2):396‐405. - PubMed
Van Marter 2000
-
- Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 2000;105(6):1194‐201. - PubMed
Villar 1995
-
- Villar J, Carroli G, Belizán JM. Predictive ability of meta‐analyses of randomised controlled trials. Lancet 1995;345(8952):772‐6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

