Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions
- PMID: 27976409
- PMCID: PMC5516238
- DOI: 10.1002/bdd.2052
Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions
Abstract
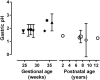
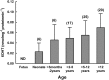
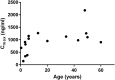
The dissolution, intestinal absorption and presystemic metabolism of a drug depend on its physicochemical characteristics but also on numerous physiological (e.g. gastrointestinal pH, volume, transit time, morphology) and biochemical factors (e.g. luminal enzymes and flora, intestinal wall enzymes and transporters). Over the past decade, evidence has accumulated indicating that these factors may differ in children and adults resulting in age-related changes in drug exposure and drug response. Thus, drug dosage may require adjustment for the pediatric population to ensure the desired therapeutic outcome and to avoid side-effects. Although tremendous progress has been made in understanding the effects of age on intestinal physiology and function, significant knowledge gaps remain. Studying and predicting pharmacokinetics in pediatric patients remains challenging due to ethical concerns associated with clinical trials in this vulnerable population, and because of the paucity of predictive in vitro and in vivo animal assays. This review details the current knowledge related to developmental changes determining intestinal drug absorption and pre-systemic metabolism. Supporting experimental approaches as well as physiologically based pharmacokinetic modeling are also discussed together with their limitations and challenges. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: intestinal absorption; ontogeny; pediatrics; physiologically based pharmacokinetic modeling.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures

References
-
- Christensen ML. Best pharmaceuticals for children act and pediatric research equity act: time for permanent status. J Pediatr Pharmacol Ther 2012; 17: 140–141. doi:10.5863/1551-6776-17.2.140. - DOI - PMC - PubMed
-
- Shirkey H. Therapeutic orphans. J Pediatr 1968; 72: 119–120. - PubMed
-
- Kimland E, Odlind V. Off‐label drug use in pediatric patients. Clin Pharmacol Ther 2012; 91: 796–801 .DOI: clpt201226 [pii];10.1038/clpt.2012.26 [doi] - PubMed
-
- Standing JF, Tuleu C. Paediatric formulations – getting to the heart of the problem. Int J Pharm 2005; 300: 56–66 .DOI: S0378–5173(05)00313–3 [pii];10.1016/j.ijpharm.2005.05.006 [doi] - PubMed
-
- Sutherland JM. Fatal cardiovascular collapse of infants receiving large amounts of chloramphenicol. AMA J Dis Child 1959; 97: 761–767. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

