Disentangling the influence of earthworms in sugarcane rhizosphere
- PMID: 27976685
- PMCID: PMC5156904
- DOI: 10.1038/srep38923
Disentangling the influence of earthworms in sugarcane rhizosphere
Abstract
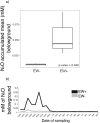
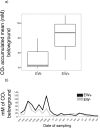
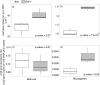
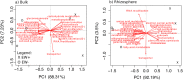
For the last 150 years many studies have shown the importance of earthworms for plant growth, but the exact mechanisms involved in the process are still poorly understood. Many important functions required for plant growth can be performed by soil microbes in the rhizosphere. To investigate earthworm influence on the rhizosphere microbial community, we performed a macrocosm experiment with and without Pontoscolex corethrurus (EW+ and EW-, respectively) and followed various soil and rhizosphere processes for 217 days with sugarcane. In EW+ treatments, N2O concentrations belowground (15 cm depth) and relative abundances of nitrous oxide genes (nosZ) were higher in bulk soil and rhizosphere, suggesting that soil microbes were able to consume earthworm-induced N2O. Shotgun sequencing (total DNA) revealed that around 70 microbial functions in bulk soil and rhizosphere differed between EW+ and EW- treatments. Overall, genes indicative of biosynthetic pathways and cell proliferation processes were enriched in EW+ treatments, suggesting a positive influence of worms. In EW+ rhizosphere, functions associated with plant-microbe symbiosis were enriched relative to EW- rhizosphere. Ecological networks inferred from the datasets revealed decreased niche diversification and increased keystone functions as an earthworm-derived effect. Plant biomass was improved in EW+ and worm population proliferated.
Figures


References
-
- Bouché M. B. Action de la faune sur les état de la matière organique dans les écosystèmes in Humification et Biodegradation (eds. Kilbertus G., Reisinger O., Mourey A. & Canela da Fonseca) 157–168 (Sarreguemines: Pierron, 1975).
-
- Lavelle P. Functional domains in soils. Ecol. Res. 17, 441–450 (2002).
-
- James S. W. Soil nitrogen, phosphorus, and organic matter processing by earthworms in tallgrass prairie. Ecology 72, 2101–2109 (1991).
-
- Lavelle P., Melendez G., Pashanasi B. & Schaefer R. Nitrogen mineralization and reorganization in casts of the geophagous tropical earthworm Pontoscolex corethrurus (Glossoscolecidae). Biol. Fert. Soils 14, 49–53 (1992).
-
- Subler S., Baranski C. M. & Edwards C. A. Earthworm additions increased short-term nitrogen availability and leaching in two grain-crop agroecosystems. Soil Biol. Biochem. 29, 413–421 (1997).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

