Epithelial ovarian carcinoma diagnosis by desorption electrospray ionization mass spectrometry imaging
- PMID: 27976698
- PMCID: PMC5156945
- DOI: 10.1038/srep39219
Epithelial ovarian carcinoma diagnosis by desorption electrospray ionization mass spectrometry imaging
Abstract
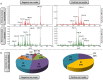
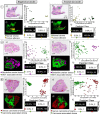
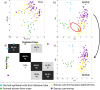
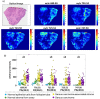
Ovarian cancer is highly prevalent among European women, and is the leading cause of gynaecological cancer death. Current histopathological diagnoses of tumour severity are based on interpretation of, for example, immunohistochemical staining. Desorption electrospray mass spectrometry imaging (DESI-MSI) generates spatially resolved metabolic profiles of tissues and supports an objective investigation of tumour biology. In this study, various ovarian tissue types were analysed by DESI-MSI and co-registered with their corresponding haematoxylin and eosin (H&E) stained images. The mass spectral data reveal tissue type-dependent lipid profiles which are consistent across the n = 110 samples (n = 107 patients) used in this study. Multivariate statistical methods were used to classify samples and identify molecular features discriminating between tissue types. Three main groups of samples (epithelial ovarian carcinoma, borderline ovarian tumours, normal ovarian stroma) were compared as were the carcinoma histotypes (serous, endometrioid, clear cell). Classification rates >84% were achieved for all analyses, and variables differing statistically between groups were determined and putatively identified. The changes noted in various lipid types help to provide a context in terms of tumour biochemistry. The classification of unseen samples demonstrates the capability of DESI-MSI to characterise ovarian samples and to overcome existing limitations in classical histopathology.
Figures

References
-
- Kommoss S. et al.. Specialized pathology review in patients with ovarian cancer: results from a prospective study. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society 23, 1376–1382, doi: 10.1097/IGC.0b013e3182a01813 (2013). - DOI - PubMed
-
- Leung F., Musrap N., Diamandis E. P. & Kulasingam V. Advances in mass spectrometry-based technologies to direct personalized medicine in ovarian cancer. Advances in Integrative Medicine 1, 74–86, 10.1016/j.trprot.2013.08.001 (2013). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

