Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement
- PMID: 27976722
- PMCID: PMC5171775
- DOI: 10.1038/ncomms13697
Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement
Abstract
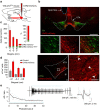
In addition to dopamine neurons, the ventral tegmental area (VTA) contains GABA-, glutamate- and co-releasing neurons, and recent reports suggest a complex role for the glutamate neurons in behavioural reinforcement. We report that optogenetic stimulation of VTA glutamate neurons or terminals serves as a positive reinforcer on operant behavioural assays. Mice display marked preference for brief over sustained VTA glutamate neuron stimulation resulting in behavioural responses that are notably distinct from dopamine neuron stimulation and resistant to dopamine receptor antagonists. Whole-cell recordings reveal EPSCs following stimulation of VTA glutamate terminals in the nucleus accumbens or local VTA collaterals; but reveal both excitatory and monosynaptic inhibitory currents in the ventral pallidum and lateral habenula, though the net effects on postsynaptic firing in each region are consistent with the observed rewarding behavioural effects. These data indicate that VTA glutamate neurons co-release GABA in a projection-target-dependent manner and that their transient activation drives positive reinforcement.
Figures





References
-
- Fields H. L., Hjelmstad G. O., Margolis E. B. & Nicola S. M. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 30, 289–316 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

