Multivoxel neurofeedback selectively modulates confidence without changing perceptual performance
- PMID: 27976739
- PMCID: PMC5171844
- DOI: 10.1038/ncomms13669
Multivoxel neurofeedback selectively modulates confidence without changing perceptual performance
Abstract
A central controversy in metacognition studies concerns whether subjective confidence directly reflects the reliability of perceptual or cognitive processes, as suggested by normative models based on the assumption that neural computations are generally optimal. This view enjoys popularity in the computational and animal literatures, but it has also been suggested that confidence may depend on a late-stage estimation dissociable from perceptual processes. Yet, at least in humans, experimental tools have lacked the power to resolve these issues convincingly. Here, we overcome this difficulty by using the recently developed method of decoded neurofeedback (DecNef) to systematically manipulate multivoxel correlates of confidence in a frontoparietal network. Here we report that bi-directional changes in confidence do not affect perceptual accuracy. Further psychophysical analyses rule out accounts based on simple shifts in reporting strategy. Our results provide clear neuroscientific evidence for the systematic dissociation between confidence and perceptual performance, and thereby challenge current theoretical thinking.
Conflict of interest statement
There is a potential financial conflict of interest; one of the authors is the inventor of patents related to the neurofeedback method used in this study, and the original assignee of the patents is ATR, with which M.K. is affiliated. The remaining authors declare no competing financial interests.
Figures

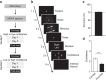
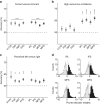
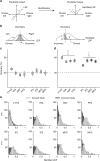
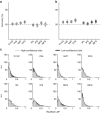




Similar articles
-
[Modulation of Metacognition with Decoded Neurofeedback].Brain Nerve. 2017 Dec;69(12):1427-1432. doi: 10.11477/mf.1416200929. Brain Nerve. 2017. PMID: 29282346 Review. Japanese.
-
Multistable Perception and the Role of the Frontoparietal Cortex in Perceptual Inference.Annu Rev Psychol. 2018 Jan 4;69:77-103. doi: 10.1146/annurev-psych-010417-085944. Epub 2017 Sep 11. Annu Rev Psychol. 2018. PMID: 28854000 Review.
-
Decoded fMRI neurofeedback can induce bidirectional confidence changes within single participants.Neuroimage. 2017 Apr 1;149:323-337. doi: 10.1016/j.neuroimage.2017.01.069. Epub 2017 Feb 3. Neuroimage. 2017. PMID: 28163140 Free PMC article. Clinical Trial.
-
Evidence accumulation during perceptual decisions in humans varies as a function of dorsal frontoparietal organization.Nat Hum Behav. 2020 Aug;4(8):844-855. doi: 10.1038/s41562-020-0863-4. Epub 2020 Apr 20. Nat Hum Behav. 2020. PMID: 32313233
-
Attention enhances multi-voxel representation of novel objects in frontal, parietal and visual cortices.Neuroimage. 2015 Apr 1;109:429-37. doi: 10.1016/j.neuroimage.2014.12.083. Epub 2015 Jan 9. Neuroimage. 2015. PMID: 25583612
Cited by
-
Modulating subjective pain perception with decoded Montreal Neurological Institute-space neurofeedback: a proof-of-concept study.Philos Trans R Soc Lond B Biol Sci. 2024 Dec 2;379(1915):20230082. doi: 10.1098/rstb.2023.0082. Epub 2024 Oct 21. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39428876
-
Overlapping and unique neural circuits are activated during perceptual decision making and confidence.Sci Rep. 2020 Nov 27;10(1):20761. doi: 10.1038/s41598-020-77820-6. Sci Rep. 2020. PMID: 33247212 Free PMC article.
-
A simulation-based approach to improve decoded neurofeedback performance.Neuroimage. 2019 Jul 15;195:300-310. doi: 10.1016/j.neuroimage.2019.03.062. Epub 2019 Apr 4. Neuroimage. 2019. PMID: 30954707 Free PMC article.
-
Alpha oscillations and stimulus-evoked activity dissociate metacognitive reports of attention, visibility, and confidence in a rapid visual detection task.J Vis. 2022 Sep 2;22(10):20. doi: 10.1167/jov.22.10.20. J Vis. 2022. PMID: 36166234 Free PMC article.
-
Towards an unconscious neural reinforcement intervention for common fears.Proc Natl Acad Sci U S A. 2018 Mar 27;115(13):3470-3475. doi: 10.1073/pnas.1721572115. Epub 2018 Mar 6. Proc Natl Acad Sci U S A. 2018. PMID: 29511106 Free PMC article.
References
-
- Terrace H. S. & Metcalfe J. The Missing Link in Cognition: Origins of Self-Reflective Consciousness Oxford Univ. Press (2005).
-
- Smith J. D., Shields W. E. & Washburn D. A. The comparative psychology of uncertainty monitoring and metacognition. Behav. Brain Sci. 26, 317–339 discussion 340–373 (2003). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

