Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling
- PMID: 27976855
- PMCID: PMC5827935
- DOI: 10.1021/acs.analchem.6b04631
Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling
Conflict of interest statement
The authors declare no competing financial interest.
Figures

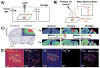

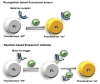
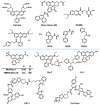
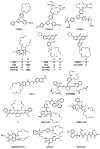



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

