Metabolism of the Tobacco Carcinogen 2-Amino-9H-pyrido[2,3-b]indole (AαC) in Primary Human Hepatocytes
- PMID: 27976871
- PMCID: PMC5439433
- DOI: 10.1021/acs.chemrestox.6b00394
Metabolism of the Tobacco Carcinogen 2-Amino-9H-pyrido[2,3-b]indole (AαC) in Primary Human Hepatocytes
Abstract
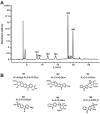
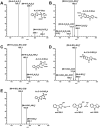
2-Amino-9H-pyrido[2,3-b]indole (AαC) is the most abundant carcinogenic heterocyclic aromatic amine (HAA) formed in mainstream tobacco smoke. AαC is a liver carcinogen in rodents, but its carcinogenic potential in humans is not known. To obtain a better understanding of the genotoxicity of AαC in humans, we have investigated its metabolism and its ability to form DNA adducts in human hepatocytes. Primary human hepatocytes were treated with AαC at doses ranging from 0.1-50 μM, and the metabolites were characterized by ultra-performance LC/ion trap multistage mass spectrometry (UPLC/MSn). Six major metabolites were identified: a ring-oxidized doubly conjugated metabolite, N2-acetyl-2-amino-9H-pyrido[2,3-b]indole-6-yl-oxo-(β-d-glucuronic acid) (N2-acetyl-AαC-6-O-Gluc); two ring-oxidized glucuronide (Gluc) conjugates: 2-amino-9H-pyrido[2,3-b]indol-3-yl-oxo-(β-d-glucuronic acid) (AαC-3-O-Gluc) and 2-amino-9H-pyrido[2,3-b]indol-6-yl-oxo-(β-d-glucuronic acid) (AαC-6-O-Gluc); two sulfate conjugates, 2-amino-9H-pyrido[2,3-b]indol-3-yl sulfate (AαC-3-O-SO3H) and 2-amino-9H-pyrido[2,3-b]indol-6-yl sulfate (AαC-6-O-SO3H); and the Gluc conjugate, N2-(β-d-glucosidurony1)-2-amino-9H-pyrido[2,3-b]indole (AαC-N2-Gluc). In addition, four minor metabolites were identified: N2-acetyl-9H-pyrido[2,3-b]indol-3-yl sulfate (N2-acetyl-AαC-3-O-SO3H), N2-acetyl-9H-pyrido[2,3-b]indol-6-yl sulfate (N2-acetyl-AαC-6-O-SO3H), N2-acetyl-2-amino-9H-pyrido[2,3-b]indol-3-yl-oxo-(β-d-glucuronic acid) (N2-acetyl-AαC-3-O-Gluc), and O-(β-d-glucosidurony1)-2-hydroxyamino-9H-pyrido[2,3-b]indole (AαC-HN2-O-Gluc). The latter metabolite, AαC-HN2-O-Gluc is a reactive intermediate that binds to DNA to form the covalent adduct N-(2'-deoxyguanosin-8-yl)-2-amino-9H-pyrido[2,3-b]indole (dG-C8-AαC). Preincubation of hepatocytes with furafylline, a selective mechanism-based inhibitor of P450 1A2, resulted in a strong decrease in the formation of AαC-HN2-O-Gluc and a concomitant decrease in DNA adduct formation. Our findings describe the major pathways of metabolism of AαC in primary human hepatocytes and reveal the importance of N-acetylation and glucuronidation in metabolism of AαC. P450 1A2 is a major isoform involved in the bioactivation of AαC to form the reactive AαC-HN2-O-Gluc conjugate and AαC-DNA adducts.
Figures
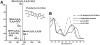


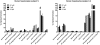
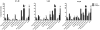
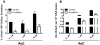
References
-
- Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:725–731. - PubMed
-
- Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

