Characterization of Two Late-Stage Enzymes Involved in Fosfomycin Biosynthesis in Pseudomonads
- PMID: 27977135
- PMCID: PMC5315633
- DOI: 10.1021/acschembio.6b00939
Characterization of Two Late-Stage Enzymes Involved in Fosfomycin Biosynthesis in Pseudomonads
Abstract
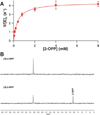
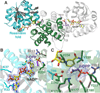
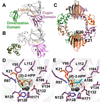
The broad-spectrum phosphonate antibiotic fosfomycin is currently in use for clinical treatment of infections caused by both Gram-positive and Gram-negative uropathogens. The antibiotic is biosynthesized by various streptomycetes, as well as by pseudomonads. Notably, the biosynthetic strategies used by the two genera share only two steps: the first step in which primary metabolite phosphoenolpyruvate (PEP) is converted to phosphonopyruvate (PnPy) and the terminal step in which 2-hydroxypropylphosphonate (2-HPP) is converted to fosfomycin. Otherwise, distinct enzymatic paths are employed. Here, we biochemically confirm the last two steps in the fosfomycin biosynthetic pathway of Pseudomonas syringae PB-5123, showing that Psf3 performs the reduction of 2-oxopropylphosphonate (2-OPP) to (S)-2-HPP, followed by the Psf4-catalyzed epoxidation of (S)-2-HPP to fosfomycin. Psf4 can also accept (R)-2-HPP as a substrate but instead performs an oxidation to make 2-OPP. We show that the combined activities of Psf3 and Psf4 can be used to convert racemic 2-HPP to fosfomycin in an enantioconvergent process. X-ray structures of each enzyme with bound substrates provide insights into the stereospecificity of each conversion. These studies shed light on the reaction mechanisms of the two terminal enzymes in a distinct pathway employed by pseudomonads for the production of a potent antimicrobial agent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Biosynthesis of fosfomycin in pseudomonads reveals an unexpected enzymatic activity in the metallohydrolase superfamily.Proc Natl Acad Sci U S A. 2021 Jun 8;118(23):e2019863118. doi: 10.1073/pnas.2019863118. Proc Natl Acad Sci U S A. 2021. PMID: 34074759 Free PMC article.
-
Different biosynthetic pathways to fosfomycin in Pseudomonas syringae and Streptomyces species.Antimicrob Agents Chemother. 2012 Aug;56(8):4175-83. doi: 10.1128/AAC.06478-11. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615277 Free PMC article.
-
Purification and characterization of the epoxidase catalyzing the formation of fosfomycin from Pseudomonas syringae.Biochemistry. 2008 Aug 19;47(33):8726-35. doi: 10.1021/bi800877v. Epub 2008 Jul 26. Biochemistry. 2008. PMID: 18656958 Free PMC article.
-
Biosynthetic pathways and enzymes involved in the production of phosphonic acid natural products.Biosci Biotechnol Biochem. 2021 Jan 7;85(1):42-52. doi: 10.1093/bbb/zbaa052. Biosci Biotechnol Biochem. 2021. PMID: 33577658 Review.
-
Fosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotherapy. 2014 Aug;34(8):845-57. doi: 10.1002/phar.1434. Epub 2014 Apr 30. Pharmacotherapy. 2014. PMID: 24782335 Review.
Cited by
-
Biosynthesis of fosfomycin in pseudomonads reveals an unexpected enzymatic activity in the metallohydrolase superfamily.Proc Natl Acad Sci U S A. 2021 Jun 8;118(23):e2019863118. doi: 10.1073/pnas.2019863118. Proc Natl Acad Sci U S A. 2021. PMID: 34074759 Free PMC article.
-
Engineering non-haem iron enzymes for enantioselective C(sp3)-F bond formation via radical fluorine transfer.Nat Synth. 2024 Aug;3(8):958-966. doi: 10.1038/s44160-024-00507-7. Epub 2024 Mar 28. Nat Synth. 2024. PMID: 39364063 Free PMC article.
-
The intriguing biology and chemistry of fosfomycin: the only marketed phosphonate antibiotic.RSC Adv. 2019 Dec 19;9(72):42204-42218. doi: 10.1039/c9ra08299a. eCollection 2019 Dec 18. RSC Adv. 2019. PMID: 35548698 Free PMC article. Review.
-
Convergent and divergent biosynthetic strategies towards phosphonic acid natural products.Curr Opin Chem Biol. 2022 Dec;71:102214. doi: 10.1016/j.cbpa.2022.102214. Epub 2022 Oct 3. Curr Opin Chem Biol. 2022. PMID: 36202046 Free PMC article. Review.
-
Stereochemical and Mechanistic Investigation of the Reaction Catalyzed by Fom3 from Streptomyces fradiae, a Cobalamin-Dependent Radical S-Adenosylmethionine Methylase.Biochemistry. 2018 Aug 21;57(33):4972-4984. doi: 10.1021/acs.biochem.8b00693. Epub 2018 Aug 9. Biochemistry. 2018. PMID: 30036047 Free PMC article.
References
-
- Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2010;65:1862–1877. - PubMed
-
- Christensen BG, Leanza WJ, Beattie TR, Patchett AA, Arison BH, Ormond RE, Kuehl FA, Jr, Albers-Schonberg G, Jardetzky O. Phosphonomycin: structure and synthesis. Science. 1969;166:123–125. - PubMed
-
- Kahan FM, Kahan JS, Cassidy PJ, Kropp H. The mechanism of action of fosfomycin (phosphonomycin) Ann. N. Y. Acad. Sci. 1974;235:364–386. - PubMed
-
- Marquardt JL, Brown ED, Lane WS, Haley TM, Ichikawa Y, Wong CH, Walsh CT. Kinetics, stoichiometry, and identification of the reactive thiolate in the inactivation of UDP-GlcNAc enolpyruvoyl transferase by the antibiotic fosfomycin. Biochemistry. 1994;33:10646–10651. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

