Development of a Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo): Study Protocol
- PMID: 27977390
- PMCID: PMC5200844
- DOI: 10.2196/resprot.6558
Development of a Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo): Study Protocol
Abstract
Background: Individual pharmacokinetic assessment is a critical component of tailored prophylaxis for hemophilia patients. Population pharmacokinetics allows using individual sparse data, thus simplifying individual pharmacokinetic studies. Implementing population pharmacokinetics capacity for the hemophilia community is beyond individual reach and requires a system effort.
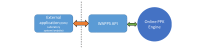
Objective: The Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo) project aims to assemble a database of patient pharmacokinetic data for all existing factor concentrates, develop and validate population pharmacokinetics models, and integrate these models within a Web-based calculator for individualized pharmacokinetic estimation in patients at participating treatment centers.
Methods: Individual pharmacokinetic studies on factor VIII and IX concentrates will be sourced from pharmaceutical companies and independent investigators. All factor concentrate manufacturers, hemophilia treatment centers (HTCs), and independent investigators (identified via a systematic review of the literature) having on file pharmacokinetic data and willing to contribute full or sparse pharmacokinetic data will be eligible for participation. Multicompartmental modeling will be performed using a mixed-model approach for derivation and Bayesian forecasting for estimation of individual sparse data. NONMEM (ICON Development Solutions) will be used as modeling software.
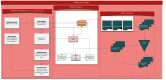
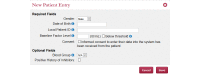
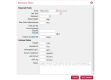
Results: The WAPPS-Hemo research network has been launched and is currently joined by 30 HTCs from across the world. We have gathered dense individual pharmacokinetic data on 878 subjects, including several replicates, on 21 different molecules from 17 different sources. We have collected sparse individual pharmacokinetic data on 289 subjects from the participating centers through the testing phase of the WAPPS-Hemo Web interface. We have developed prototypal population pharmacokinetics models for 11 molecules. The WAPPS-Hemo website (available at www.wapps-hemo.org, version 2.4), with core functionalities allowing hemophilia treaters to obtain individual pharmacokinetic estimates on sparse data points after 1 or more infusions of a factor concentrate, was launched for use within the research network in July 2015.
Conclusions: The WAPPS-Hemo project and research network aims to make it easier to perform individual pharmacokinetic assessments on a reduced number of plasma samples by adoption of a population pharmacokinetics approach. The project will also gather data to substantially enhance the current knowledge about factor concentrate pharmacokinetics and sources of its variability in target populations.
Trial registration: ClinicalTrials.gov NCT02061072; https://clinicaltrials.gov/ct2/show/NCT02061072 (Archived by WebCite at http://www.webcitation.org/6mRK9bKP6).
Keywords: factor IX; factor VIII; hemophilia; population pharmacokinetics; tailored prophylaxis.
©Alfonso Iorio, Arun Keepanasseril, Gary Foster, Tamara Navarro-Ruan, Alanna McEneny-King, Andrea N Edginton, Lehana Thabane. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 15.12.2016.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Feldman BM, Pai M, Rivard GE, Israels S, Poon M, Demers C, Robinson S, Luke K, Wu JK, Gill K, Lillicrap D, Babyn P, McLimont M, Blanchette VS, Association of Hemophilia Clinic Directors of Canada Prophylaxis Study Group Tailored prophylaxis in severe hemophilia A: interim results from the first 5 years of the Canadian Hemophilia Primary Prophylaxis Study. J Thromb Haemost. 2006 Jun;4(6):1228–1236. doi: 10.1111/j.1538-7836.2006.01953.x. http://dx.doi.org/10.1111/j.1538-7836.2006.01953.x - DOI - DOI - PubMed
-
- Risebrough N, Oh P, Blanchette V, Curtin J, Hitzler J, Feldman BM. Cost-utility analysis of Canadian tailored prophylaxis, primary prophylaxis and on-demand therapy in young children with severe haemophilia A. Haemophilia. 2008 Jul;14(4):743–752. doi: 10.1111/j.1365-2516.2008.01664.x. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

