Unique Function of the Bacterial Chromosome Segregation Machinery in Apically Growing Streptomyces - Targeting the Chromosome to New Hyphal Tubes and its Anchorage at the Tips
- PMID: 27977672
- PMCID: PMC5157956
- DOI: 10.1371/journal.pgen.1006488
Unique Function of the Bacterial Chromosome Segregation Machinery in Apically Growing Streptomyces - Targeting the Chromosome to New Hyphal Tubes and its Anchorage at the Tips
Abstract
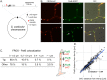
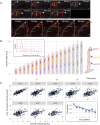
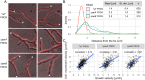
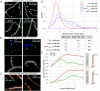
The coordination of chromosome segregation with cell growth is fundamental to the proliferation of any organism. In most unicellular bacteria, chromosome segregation is strictly coordinated with cell division and involves ParA that moves the ParB nucleoprotein complexes bi- or unidirectionally toward the cell pole(s). However, the chromosome organization in multiploid, apically extending and branching Streptomyces hyphae challenges the known mechanisms of bacterial chromosome segregation. The complex Streptomyces life cycle involves two stages: vegetative growth and sporulation. In the latter stage, multiple cell divisions accompanied by chromosome compaction and ParAB assisted segregation turn multigenomic hyphal cell into a chain of unigenomic spores. However, the requirement for active chromosome segregation is unclear in the absence of canonical cell division during vegetative growth except in the process of branch formation. The mechanism by which chromosomes are targeted to new hyphae in streptomycete vegetative growth has remained unknown until now. Here, we address the question of whether active chromosome segregation occurs at this stage. Applied for the first time in Streptomyces, labelling of the chromosomal replication initiation region (oriC) and time-lapse microscopy, revealed that in vegetative hyphae every copy of the chromosome is complexed with ParB, whereas ParA, through interaction with the apical protein complex (polarisome), tightly anchors only one chromosome at the hyphal tip. The anchor is maintained during replication, when ParA captures one of the daughter oriCs. During spore germination and branching, ParA targets one of the multiple chromosomal copies to the new hyphal tip, enabling efficient elongation of hyphal tube. Thus, our studies reveal a novel role for ParAB proteins during hyphal tip establishment and extension.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Compaction and control-the role of chromosome-organizing proteins in Streptomyces.FEMS Microbiol Rev. 2020 Nov 24;44(6):725-739. doi: 10.1093/femsre/fuaa028. FEMS Microbiol Rev. 2020. PMID: 32658291 Free PMC article. Review.
-
ParA and ParB coordinate chromosome segregation with cell elongation and division during Streptomyces sporulation.Open Biol. 2016 Apr;6(4):150263. doi: 10.1098/rsob.150263. Epub 2016 Apr 27. Open Biol. 2016. PMID: 27248800 Free PMC article.
-
[Chromosome segregation and cell division during the growth and differentiation of Streptomyces].Postepy Hig Med Dosw (Online). 2007 Oct 12;61:565-75. Postepy Hig Med Dosw (Online). 2007. PMID: 17971760 Review. Polish.
-
Topoisomerase I (TopA) is recruited to ParB complexes and is required for proper chromosome organization during Streptomyces coelicolor sporulation.J Bacteriol. 2013 Oct;195(19):4445-55. doi: 10.1128/JB.00798-13. Epub 2013 Aug 2. J Bacteriol. 2013. PMID: 23913317 Free PMC article.
-
SMC protein-dependent chromosome condensation during aerial hyphal development in Streptomyces.J Bacteriol. 2009 Jan;191(1):310-9. doi: 10.1128/JB.00513-08. Epub 2008 Oct 17. J Bacteriol. 2009. PMID: 18931116 Free PMC article.
Cited by
-
Binary or Nonbinary Fission? Reproductive Mode of a Predatory Bacterium Depends on Prey Size.mBio. 2023 Jun 27;14(3):e0077223. doi: 10.1128/mbio.00772-23. Epub 2023 May 10. mBio. 2023. PMID: 37162334 Free PMC article.
-
Bacterial Cell Division: Nonmodels Poised to Take the Spotlight.Annu Rev Microbiol. 2017 Sep 8;71:393-411. doi: 10.1146/annurev-micro-102215-095657. Epub 2017 Jul 11. Annu Rev Microbiol. 2017. PMID: 28697666 Free PMC article. Review.
-
Formation of wall-less cells in Kitasatospora viridifaciens requires cytoskeletal protein FilP in oxygen-limiting conditions.Mol Microbiol. 2021 Jun;115(6):1181-1190. doi: 10.1111/mmi.14662. Epub 2020 Dec 19. Mol Microbiol. 2021. PMID: 33278050 Free PMC article.
-
Compaction and control-the role of chromosome-organizing proteins in Streptomyces.FEMS Microbiol Rev. 2020 Nov 24;44(6):725-739. doi: 10.1093/femsre/fuaa028. FEMS Microbiol Rev. 2020. PMID: 32658291 Free PMC article. Review.
-
Where and When Bacterial Chromosome Replication Starts: A Single Cell Perspective.Front Microbiol. 2018 Nov 26;9:2819. doi: 10.3389/fmicb.2018.02819. eCollection 2018. Front Microbiol. 2018. PMID: 30534115 Free PMC article. Review.
References
-
- Mierzejewska J, Jagura-Burdzy G. Prokaryotic ParA-ParB-parS system links bacterial chromosome segregation with the cell cycle. Plasmid. 2012. pp. 1–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

