Modulating the Biologic Activity of Mesenteric Lymph after Traumatic Shock Decreases Systemic Inflammation and End Organ Injury
- PMID: 27977787
- PMCID: PMC5158049
- DOI: 10.1371/journal.pone.0168322
Modulating the Biologic Activity of Mesenteric Lymph after Traumatic Shock Decreases Systemic Inflammation and End Organ Injury
Abstract
Introduction: Trauma/hemorrhagic shock (T/HS) causes the release of pro-inflammatory mediators into the mesenteric lymph (ML), triggering a systemic inflammatory response and acute lung injury (ALI). Direct and pharmacologic vagal nerve stimulation prevents gut barrier failure and alters the biologic activity of ML after injury. We hypothesize that treatment with a pharmacologic vagal agonist after T/HS would attenuate the biologic activity of ML and prevent ALI.
Methods: ML was collected from male Sprague-Dawley rats after T/HS, trauma-sham shock (T/SS) or T/HS with administration of the pharmacologic vagal agonist CPSI-121. ML samples from each experimental group were injected into naïve mice to assess biologic activity. Blood samples were analyzed for changes in STAT3 phosphorylation (pSTAT3). Lung injury was characterized by histology, permeability and immune cell recruitment.
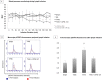
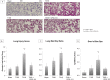
Results: T/HS lymph injected in naïve mice caused a systemic inflammatory response characterized by hypotension and increased circulating monocyte pSTAT3 activity. Injection of T/HS lymph also resulted in ALI, confirmed by histology, lung permeability and increased recruitment of pulmonary macrophages and neutrophils to lung parenchyma. CPSI-121 attenuated T/HS lymph-induced systemic inflammatory response and ALI with stable hemodynamics and similar monocyte pSTAT3 levels, lung histology, lung permeability and lung immune cell recruitment compared to animals injected with lymph from T/SS.
Conclusion: Treatment with CPSI-121 after T/HS attenuated the biologic activity of the ML and decreased ALI. Given the superior clinical feasibility of utilizing a pharmacologic approach to vagal nerve stimulation, CPSI-121 is a potential treatment strategy to limit end organ dysfunction after injury.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A pharmacologic approach to vagal nerve stimulation prevents mesenteric lymph toxicity after hemorrhagic shock.J Trauma Acute Care Surg. 2015 Jan;78(1):52-8; discussion 58-9. doi: 10.1097/TA.0000000000000489. J Trauma Acute Care Surg. 2015. PMID: 25539203
-
Amiloride moderates increased gut permeability and diminishes mesenteric lymph-mediated priming of neutrophils in trauma/hemorrhagic shock.Surgery. 2006 Nov;140(5):810-7. doi: 10.1016/j.surg.2006.03.003. Epub 2006 Aug 28. Surgery. 2006. PMID: 17084725
-
Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4.FASEB J. 2018 Jan;32(1):97-110. doi: 10.1096/fj.201700488R. Epub 2017 Aug 29. FASEB J. 2018. PMID: 28855278
-
Role of the gut lymphatic system in multiple organ failure.Curr Opin Crit Care. 2001 Apr;7(2):92-8. doi: 10.1097/00075198-200104000-00007. Curr Opin Crit Care. 2001. PMID: 11373517 Review.
-
Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock.Front Biosci (Landmark Ed). 2015 Jun 1;20(6):927-33. doi: 10.2741/4347. Front Biosci (Landmark Ed). 2015. PMID: 25961533 Review.
Cited by
-
Gut integrity in critical illness.J Intensive Care. 2019 Mar 20;7:17. doi: 10.1186/s40560-019-0372-6. eCollection 2019. J Intensive Care. 2019. PMID: 30923621 Free PMC article. Review.
-
Vagus Nerve Stimulation and the Cardiovascular System.Cold Spring Harb Perspect Med. 2020 Feb 3;10(2):a034173. doi: 10.1101/cshperspect.a034173. Cold Spring Harb Perspect Med. 2020. PMID: 31109966 Free PMC article. Review.
-
Innate immune responses to trauma.Nat Immunol. 2018 Apr;19(4):327-341. doi: 10.1038/s41590-018-0064-8. Epub 2018 Mar 5. Nat Immunol. 2018. PMID: 29507356 Free PMC article. Review.
-
ASK1-p38 cascaded signal mediates pulmonary microvascular endothelial barrier injury induced by the return of PHSML in rats.RSC Adv. 2019 Feb 8;9(9):4870-4875. doi: 10.1039/c8ra08473d. eCollection 2019 Feb 5. RSC Adv. 2019. PMID: 35514647 Free PMC article.
-
Upregulation of proBDNF in the Mesenteric Lymph Nodes in Septic Mice.Neurotox Res. 2019 Oct;36(3):540-550. doi: 10.1007/s12640-019-00081-3. Epub 2019 Jul 5. Neurotox Res. 2019. PMID: 31278527
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

