Disrupting Mosquito Reproduction and Parasite Development for Malaria Control
- PMID: 27977810
- PMCID: PMC5158081
- DOI: 10.1371/journal.ppat.1006060
Disrupting Mosquito Reproduction and Parasite Development for Malaria Control
Abstract
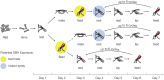
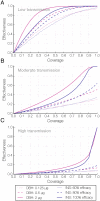
The control of mosquito populations with insecticide treated bed nets and indoor residual sprays remains the cornerstone of malaria reduction and elimination programs. In light of widespread insecticide resistance in mosquitoes, however, alternative strategies for reducing transmission by the mosquito vector are urgently needed, including the identification of safe compounds that affect vectorial capacity via mechanisms that differ from fast-acting insecticides. Here, we show that compounds targeting steroid hormone signaling disrupt multiple biological processes that are key to the ability of mosquitoes to transmit malaria. When an agonist of the steroid hormone 20-hydroxyecdysone (20E) is applied to Anopheles gambiae females, which are the dominant malaria mosquito vector in Sub Saharan Africa, it substantially shortens lifespan, prevents insemination and egg production, and significantly blocks Plasmodium falciparum development, three components that are crucial to malaria transmission. Modeling the impact of these effects on Anopheles population dynamics and Plasmodium transmission predicts that disrupting steroid hormone signaling using 20E agonists would affect malaria transmission to a similar extent as insecticides. Manipulating 20E pathways therefore provides a powerful new approach to tackle malaria transmission by the mosquito vector, particularly in areas affected by the spread of insecticide resistance.
Conflict of interest statement
A patent application covering some aspects of this work has been filed on behalf of FC, PG, EGK and DP by Harvard University.
Figures


Similar articles
-
20-Hydroxyecdysone (20E) signaling as a promising target for the chemical control of malaria vectors.Parasit Vectors. 2021 Jan 29;14(1):86. doi: 10.1186/s13071-020-04558-5. Parasit Vectors. 2021. PMID: 33514413 Free PMC article. Review.
-
Current vector control challenges in the fight against malaria.Acta Trop. 2017 Oct;174:91-96. doi: 10.1016/j.actatropica.2017.06.028. Epub 2017 Jul 3. Acta Trop. 2017. PMID: 28684267 Review.
-
A steroid hormone agonist reduces female fitness in insecticide-resistant Anopheles populations.Insect Biochem Mol Biol. 2020 Jun;121:103372. doi: 10.1016/j.ibmb.2020.103372. Epub 2020 Apr 8. Insect Biochem Mol Biol. 2020. PMID: 32276112 Free PMC article.
-
Modelling the impact of insecticide-based control interventions on the evolution of insecticide resistance and disease transmission.Parasit Vectors. 2018 Aug 28;11(1):482. doi: 10.1186/s13071-018-3025-z. Parasit Vectors. 2018. PMID: 30153869 Free PMC article.
-
Evaluating the sterilizing effect of pyriproxyfen treated mosquito nets against Anopheles gambiae at different blood-feeding intervals.Acta Trop. 2015 Oct;150:131-5. doi: 10.1016/j.actatropica.2015.07.011. Epub 2015 Jul 19. Acta Trop. 2015. PMID: 26200789
Cited by
-
Effect of fluralaner on the biology, survival, and reproductive fitness of the neotropical malaria vector Anopheles aquasalis.Malar J. 2023 Nov 7;22(1):337. doi: 10.1186/s12936-023-04767-0. Malar J. 2023. PMID: 37936198 Free PMC article.
-
Steroid hormone regulation of innate immunity in Drosophila melanogaster.PLoS Genet. 2023 Jun 15;19(6):e1010782. doi: 10.1371/journal.pgen.1010782. eCollection 2023 Jun. PLoS Genet. 2023. PMID: 37319172 Free PMC article. Review.
-
Vector biology meets disease control: using basic research to fight vector-borne diseases.Nat Microbiol. 2019 Jan;4(1):20-34. doi: 10.1038/s41564-018-0214-7. Epub 2018 Aug 27. Nat Microbiol. 2019. PMID: 30150735 Free PMC article. Review.
-
A malaria parasite phospholipid flippase safeguards midgut traversal of ookinetes for mosquito transmission.Sci Adv. 2021 Jul 23;7(30):eabf6015. doi: 10.1126/sciadv.abf6015. Print 2021 Jul. Sci Adv. 2021. PMID: 34301597 Free PMC article.
-
The ecdysone receptor regulates several key physiological factors in Anopheles funestus.Malar J. 2022 Mar 19;21(1):97. doi: 10.1186/s12936-022-04123-8. Malar J. 2022. PMID: 35305668 Free PMC article.
References
-
- WHO. World Malaria Report. World Health Organization; 2014.
-
- Harrison G. Mosquitoes, Malaria, and Man: A History of the Hostilities Since 1880. London, UK: John Murray; 1978.
-
- Zaim M, Aitio A, Nakashima N. Safety of pyrethroid-treated mosquito nets. Medical and veterinary entomology. 2000;14(1):1–5. Epub 2000/04/12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

