Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial
- PMID: 27978579
- PMCID: PMC5665683
- DOI: 10.1001/jamaoncol.2016.5383
Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial
Abstract
Importance: KRAS mutations are common in pancreatic cancer, but directly targeting the KRAS protein has thus far been unsuccessful. The aim of this trial was to block the MEK and PI3K/AKT pathways downstream of the KRAS protein as an alternate treatment strategy to slow cancer growth and prolong survival. This was the first cooperative group trial to evaluate this strategy using molecularly targeted oral combination therapy for the treatment of chemotherapy-refractory pancreatic cancer.
Objective: To compare selumetinib and MK-2206 vs modified FOLFOX (mFOLFOX) in patients with metastatic pancreatic cancer for whom gemcitabine-based therapy had failed.
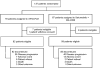
Design, setting, and participants: SWOG S1115 was a randomized phase 2 clinical trial. Between September 2012 and May 2014, 137 patients with metastatic pancreatic adenocarcinoma for whom gemcitabine-based chemotherapy had failed were randomized to selumetinib plus MK-2206 or mFOLFOX. Patients were randomized in a 1:1 fashion and stratified according to duration of prior systemic therapy and presence of liver metastases.
Interventions: Patients received selumetinib 100 mg orally per day plus MK-2206 135 mg orally once per week or mFOLFOX (oxaliplatin, 85 mg/m2 intravenous, and fluorouracil, 2400 mg/m2 intravenous infusion over 46-48 hours) on days 1 and 15 of a 28-day cycle.
Main outcomes and measures: The primary end point of the study was overall survival. Secondary objectives included evaluating toxic effects, objective tumor response, and progression-free survival.
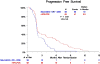
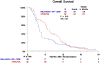
Results: There were 58 patients in the selumetinib plus MK-2206 (experimental) arm (60% male; median [range] age, 69 [54-88] years) and 62 patients in the mFOLFOX arm (35% male; median [range] age, 65 [34-82] years). In the experimental arm, median overall survival was shorter (3.9 vs 6.7 months; HR, 1.37; 95% CI, 0.90-2.08; P = .15), as was median progression-free survival (1.9 vs 2.0 months; HR, 1.61; 95% CI, 1.07-2.43; P = .02). One vs 5 patients had a partial response and 12 vs 14 patients had stable disease in the experimental arm vs mFOLFOX arm. Grade 3 or higher toxic effects were observed in 39 patients treated with selumetinib and MK-2206 vs 23 patients treated with mFOLFOX. More patients in the experimental arm discontinued therapy due to adverse events (13 vs 7 patients).
Conclusions and relevance: Dual targeting of the MEK and PI3K/AKT pathways downstream of KRAS by selumetinib plus MK-2206 did not improve overall survival in patients with metastatic pancreatic adenocarcinoma for whom gemcitabine-based chemotherapy had failed. This was the first randomized prospective evaluation of mFOLFOX in the US population that showed comparable results to CONKO-003 and PANCREOX.
Trial registration: clinicaltrials.gov Identifier: NCT01658943.
Conflict of interest statement
Stock or other ownership: Dr. Al Baghdadi (Exelixis, Array biopharma, Tracon pharmaceuticals, Cerulean and Spectrum pharmaceuticals/self); Honoraria: Drs. Chung (Celgene/self); Lowy (Pfizer/self); Philip (Celgene/self); Wang-Gillam (Axis/self); Consulting or advisory role: Drs. Al Baghdadi (Seattle genetics and Incyte/self); Chung (Celgene/self); Lowy (Merck, Halozyme/self); Philip (Celgene, Merrimack, Halozyme/self); Seery (Bayer, Halozyme/self); Wang-Gillam (Newlink, Merrimack, Pfizer/self); Speakers’ bureau: Drs. Chung (Celgene/self); Research funding: Drs. Chung (Novartis/self); Philip (Celgene, Merck/self); Tejani (Bayer/self and institution); Wang-Gilliam (Aduro, Chemocentry, CTI Biopharma, Millennium, Pfizer, Merrimack, Newlink, EMD Precision, AstraZeneca; Patent or intellectual property interest: Drs. Doyle (patent as co-discoverer of the ABCG2 (BCRP) transporter gene. The patent and the function of this gene have no bearing on the S1115 trial, and there was no conflict in participation in the trial; Lowy (Amgen/self)
Expert testimony: Dr. Lowy (Merck/self); Travel, accommodations, expenses: Dr. Al Baghdadi (Celgene and Cardinal health/self). All remaining authors have declared no conflicts of interest.
Figures
References
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(15):1960–6. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364(19):1817–25. - PubMed
-
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- UG1 CA189971/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- UG1 CA189821/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U10 CA180835/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- U10 CA180847/CA/NCI NIH HHS/United States
- U10 CA180798/CA/NCI NIH HHS/United States
- UG1 CA189809/CA/NCI NIH HHS/United States
- UG1 CA189853/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA189804/CA/NCI NIH HHS/United States
- UG1 CA189960/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- UG1 CA189822/CA/NCI NIH HHS/United States
- U10 CA180846/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- UG1 CA189954/CA/NCI NIH HHS/United States
- U10 CA180818/CA/NCI NIH HHS/United States
- U10 CA180828/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- UG1 CA189808/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- UG1 CA189953/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous