Patient-Specific Screening Using High-Grade Glioma Explants to Determine Potential Radiosensitization by a TGF-β Small Molecule Inhibitor
- PMID: 27978994
- PMCID: PMC5156509
- DOI: 10.1016/j.neo.2016.08.008
Patient-Specific Screening Using High-Grade Glioma Explants to Determine Potential Radiosensitization by a TGF-β Small Molecule Inhibitor
Abstract
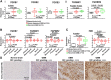
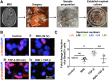
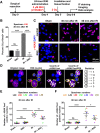
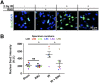
High-grade glioma (HGG), a deadly primary brain malignancy, manifests radioresistance mediated by cell-intrinsic and microenvironmental mechanisms. High levels of the cytokine transforming growth factor-β (TGF-β) in HGG promote radioresistance by enforcing an effective DNA damage response and supporting glioma stem cell self-renewal. Our analysis of HGG TCGA data and immunohistochemical staining of phosphorylated Smad2, which is the main transducer of canonical TGF-β signaling, indicated variable levels of TGF-β pathway activation across HGG tumors. These data suggest that evaluating the putative benefit of inhibiting TGF-β during radiotherapy requires personalized screening. Thus, we used explant cultures of seven HGG specimens as a rapid, patient-specific ex vivo platform to test the hypothesis that LY364947, a small molecule inhibitor of the TGF-β type I receptor, acts as a radiosensitizer in HGG. Immunofluorescence detection and image analysis of γ-H2AX foci, a marker of cellular recognition of radiation-induced DNA damage, and Sox2, a stem cell marker that increases post-radiation, indicated that LY364947 blocked these radiation responses in five of seven specimens. Collectively, our findings suggest that TGF-β signaling increases radioresistance in most, but not all, HGGs. We propose that short-term culture of HGG explants provides a flexible and rapid platform for screening context-dependent efficacy of radiosensitizing agents in patient-specific fashion. This time- and cost-effective approach could be used to personalize treatment plans in HGG patients.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Precision Medicine with TGF-β Inhibition Using Tumor Explants: Comment on "Patient-Specific Screening Using High-Grade Glioma Explants to Determine Potential Radiosensitization by a TGF-β Small Molecule Inhibitor" by N. Sumru Bayin et al.Neoplasia. 2016 Dec;18(12):806-807. doi: 10.1016/j.neo.2016.10.009. Neoplasia. 2016. PMID: 27978995 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

