Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis
- PMID: 27979356
- PMCID: PMC5181646
- DOI: 10.1016/S1474-4422(16)30291-5
Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis
Abstract
Background: Great heterogeneity exists in survival and the interval between onset of motor symptoms and dementia symptoms across synucleinopathies. We aimed to identify genetic and pathological markers that have the strongest association with these features of clinical heterogeneity in synucleinopathies.
Methods: In this retrospective study, we examined symptom onset, and genetic and neuropathological data from a cohort of patients with Lewy body disorders with autopsy-confirmed α synucleinopathy (as of Oct 1, 2015) who were previously included in other studies from five academic institutions in five cities in the USA. We used histopathology techniques and markers to assess the burden of tau neurofibrillary tangles, neuritic plaques, α-synuclein inclusions, and other pathological changes in cortical regions. These samples were graded on an ordinal scale and genotyped for variants associated with synucleinopathies. We assessed the interval from onset of motor symptoms to onset of dementia, and overall survival in groups with varying levels of comorbid Alzheimer's disease pathology according to US National Institute on Aging-Alzheimer's Association neuropathological criteria, and used multivariate regression to control for age at death and sex.
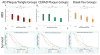
Findings: On the basis of data from 213 patients who had been followed up to autopsy and met inclusion criteria of Lewy body disorder with autopsy-confirmed α synucleinopathy, we identified 49 (23%) patients with no Alzheimer's disease neuropathology, 56 (26%) with low-level Alzheimer's disease neuropathology, 45 (21%) with intermediate-level Alzheimer's disease neuropathology, and 63 (30%) with high-level Alzheimer's disease neuropathology. As levels of Alzheimer's disease neuropathology increased, cerebral α-synuclein scores were higher, and the interval between onset of motor and dementia symptoms and disease duration was shorter (p<0·0001 for all comparisons). Multivariate regression showed independent negative associations of cerebral tau neurofibrillary tangles score with the interval between onset of motor and dementia symptoms (β -4·0, 95% CI -5·5 to -2·6; p<0·0001; R2 0·22, p<0·0001) and with survival (-2·0, -3·2 to -0·8; 0·003; 0·15, <0·0001) in models that included age at death, sex, cerebral neuritic plaque scores, cerebral α-synuclein scores, presence of cerebrovascular disease, MAPT haplotype, and APOE genotype as covariates.
Interpretation: Alzheimer's disease neuropathology is common in synucleinopathies and confers a worse prognosis for each increasing level of neuropathological change. Cerebral neurofibrillary tangles burden, in addition to α-synuclein pathology and amyloid plaque pathology, are the strongest pathological predictors of a shorter interval between onset of motor and dementia symptoms and survival. Diagnostic criteria based on reliable biomarkers for Alzheimer's disease neuropathology in synucleinopathies should help to identify the most appropriate patients for clinical trials of emerging therapies targeting tau, amyloid-β or α synuclein, and to stratify them by level of Alzheimer's disease neuropathology.
Funding: US National Institutes of Health (National Institute on Aging and National Institute of Neurological Disorders and Stroke).
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
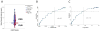

Comment in
-
Alzheimer's disease pathology in synucleinopathies.Lancet Neurol. 2017 Jan;16(1):22-23. doi: 10.1016/S1474-4422(16)30282-4. Lancet Neurol. 2017. PMID: 27979344 No abstract available.
References
-
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2007;22(12):1689–707. quiz 837. - PubMed
-
- McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72. - PubMed
-
- Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–9. - PubMed
-
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Archives of neurology. 2003;60(3):387–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

